Introduction
Dermatomyositis is a widely studied collagen disease characterized by muscle weakness and associated with skin lesions. There is a higher frequency of malignancy in patients with dermatomyositis compared to the general population. Dermatomyositis frequently presents as a paraneoplastic syndrome, and the high rates of cancer detection following its diagnosis make the screening of cancer necessary taking into account the age group and clinical presentation of each patient1-3. Additionally, dermatomyosits has been described as a paraneoplastic manifestation of gastrointestinal cancer.
The cancers most commonly associated with dermatomyositis are ovarian, pulmonary, breast, stomach, colorectal, pancreatic and non-Hodgkin’s lymphoma3-6.
Clinical case
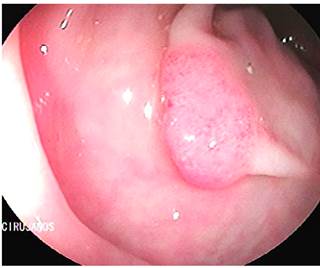
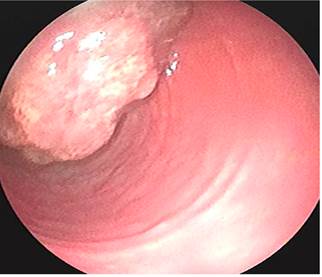
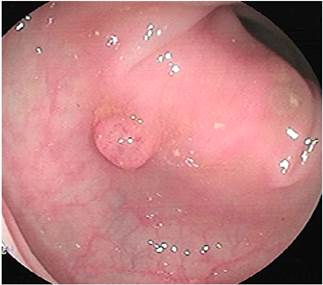
This is the case of a 58-year-old woman without any relevant history of disease and a family history of colon cancer (her sister was diagnosed with it during the fifth decade of life) who experienced an abnormal weight loss of 6 kg during the last six months associated with scanty hematochezia and occasional diarrhea episodes. Three polyps were observed on a total colonoscopy: a 7 mm polyp in the ascending colon (biopsy report: villous adenoma with low-grade dysplasia) (Figure 1), a 50 mm sessile polyp in the hepatic flexure (biopsy report: villous adenoma with low-grade dysplasia) (Figure 2) and a 30 mm polyp in the upper rectum (biopsy report: adenoma with low-grade dysplasia) (Figure 3); normal results in all 3 cell lines of the complete blood count test were reported. Endoscopic polypectomy of the rectal polyp (piecemeal resection) was performed using a hot loop, which was then classified as tubulovillous adenoma with low-grade dysplasia according to the histopathology report.
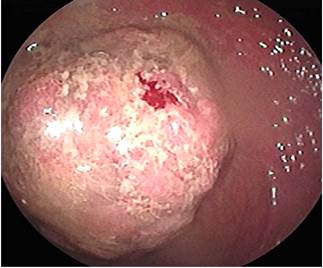
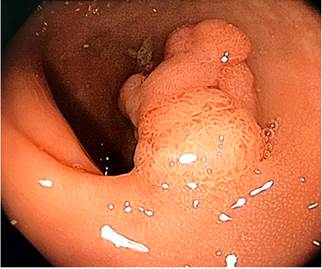
One month later, and provided that the endoscopic resection of the polyp located in the hepatic flexure was impossible, the patient underwent a right hemicolectomy and a ileotransversostomy. The following findings were described in the histopathology report of the surgical specimens resected during these procedures: 2 tubular adenomas with focal high grade dysplasia without pedicle involvement, resection margins free of lesion and absence of lymph node involvement. Two months later, in addition to postsurgical changes, a follow-up total colonoscopy allowed identifying a 40 x 20 mm sessile polyp in the transverse colon immediately distal to the anastomosis (biopsy report: villous adenoma with low-grade dysplasia) (Figure 4); due to this finding, a new surgery consisting of colectomy extension, an omentectomy and a new ileocolic anastomosis was carried out (histopathology report: tubulovillous adenoma with high-grade dysplasia and in situ adenocarcinoma immediately distal to the previous anastomosis, with no signs of infiltration, free resection margins, and no lymph node or greater omentum involvement. Six months later, a 4 mm polyp in the upper rectum was observed in a new ileocolonoscopy, no other findings were reported; the polyp was resected using a cold loop (pathology report: tubulovillous adenoma with low-grade dysplasia) (Figure 5).
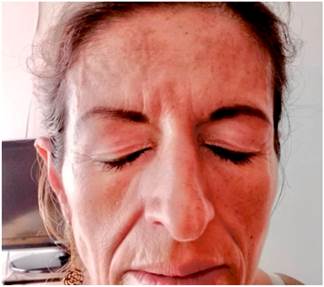
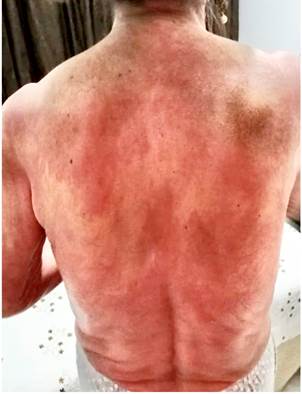
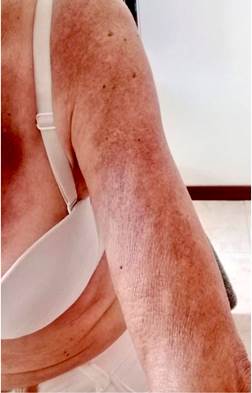
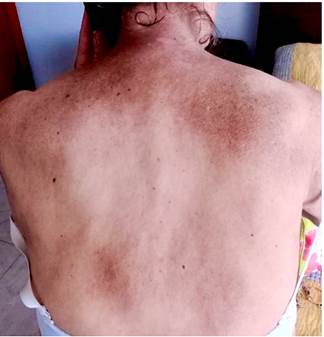
Four months before undergoing the first surgery, erythematous skin macules appeared on the patient’s chest, which became more intense and larger and extended to the upper back, the lower back, the upper limbs and the face (Figures 6-11). Two months later she developed generalized muscle weakness experiencing falls while standing or walking and being unable to get up, together with edema and distal pain in the upper and lower limbs. Initial total serum creatine kinase (CK) levels were 2028 μmol/L, however after the colectomy extension, a progressive decrease to 1800 and 1028 μmol/L, together with symptomatology improvement, was evidenced. Chronic interface dermatitis with vacuolar changes compatible with dermatomyositis was reported in the skin biopsies of the right pectoral region and right arm. Based on these clinical, laboratory and histopathological findings and behaviors, it was concluded that the patient had dermatomyositis as a paraneoplastic manifestation of colon cancer. Tumor markers (Ca 19.9 and serum CEA) were normal, and no signs suggestive of second malignancy were evidenced in both a CT scan of the chest a CT scan of the abdomen and pelvis. Currently, the patient is being monitored by the rheumatology and dermatology services; also, she is undergoing systemic steroid treatment and has reported the resolution of muscular symptoms and a significant improvement of skin lesions (Figure 12).
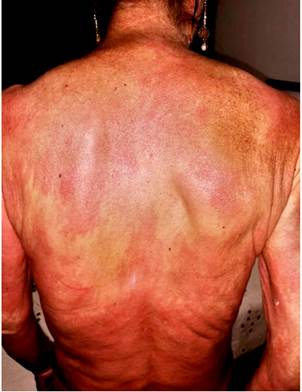
Figure 7 Erythematous plaques on the posterior cervical region, upper back and shoulders that constitute the classic “shawl sign” or capillary erythema sign.
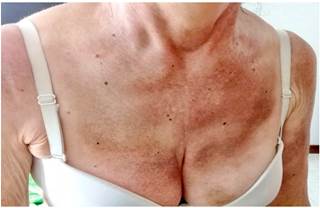
Figure 9 Violaceous erythematous plaques on the anterior triangle of the neck that extend to the chest.
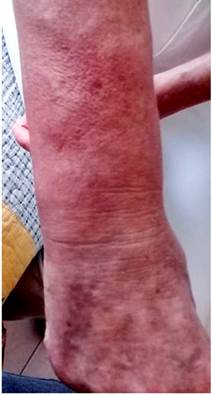
Figure 11 Distal involvement of the upper limbs caused by violaceous erythematous plaques affecting the dorsum of the hands.
Discussion
Dermatomyositis belongs to a heterogeneous group of connective tissue diseases known as idiopathic inflammatory myopathy7. Its clinical manifestations include proximal musculoskeletal weakness and inflammation, as well as skin manifestations. An incidence of 5 to 10 cases per 1 million inhabitants, being more frequent in women (3:1 ratio), has been described in the in the United States. Its peak incidence occurs between 40 and 60 years of age8. The association of dermatomyositis and malignancy was first established in 1916 in a patient with stomach cancer. Since then, multiple retrospective case series have reported this association in approximately 24% of cases9.
The etiology of cancer-related dermatomyositis remains unknown, although several hypotheses have been proposed, such as the release of bioactive components that generate immune reactions in muscle fibers and the skin10. It has been found that muscle fibers affected by autoimmune diseases overexpress specific autoantigens and that this overexpression is exhibited by tumor cells and regenerating myoblasts, which indicates that antigens expressed by both types of cells are similar11. It has been proposed that there is a crossed immune response against regenerating muscle cells, which leads to the development of dematomyositis12,13.
In 1975, Boham & Peter14 established the following diagnostic criteria for dermatomyositis: muscle weakness, high muscle enzymes, abnormal electromyography findings compatible with myopathy, compatible muscle or skin biopsy pathology report, and skin manifestations. Dermatomyositis diagnosis is made when the characteristic skin lesions are present and they are associated with 3 or more of the remaining criteria. In addition, when there is no muscle weakness, amyopathic dermatomyositis, which is also part of the same spectrum of paraneoplastic syndrome, is diagnosed16,17.
Leatham et al.18, in a retrospective study conducted in 400 patients with dermatomyositis in the United States, reported that malignancy was found in 15.8% of cases, distributed as follows: breast cancer (24.5%), hematologic cancer (17%), colorectal cancer (9.4%) and prostate cancer (9.4%). Hill et al4 found an association (in decreasing order of frequency) between the following types of cancer and dermatomyositis: ovarian, lung, stomach, colorectal, pancreatic and non-Hodgkin’s lymphoma. Sigurgeirsson et al19, in a case series conducted in 750 patients with dermatomyositis and polymyositis, reported that the most frequent cancers were colorectal and lung cancer. In Japan, Hatada et al20 found that gastric cancer was the most frequent malignancy in patients with dermatomyositis (25.4%). In all case series, cancers of the digestive tract are among the most frequent malignancies in patients with this condition. In addition, several studies have reported that people with dematomyositis have a higher risk of developing colorectal cancer4,19,21-23.
There is ambiguity in the existing literature regarding the presence of clinical or laboratory and imaging predictors of malignancy in patients with dermatomyositis. In this sense, the presence of interstitial pneumonitis, elevated serum CK levels, skin necrosis (ulcers), poikiloderma, erythrocyte sedimentation rate (ESR) > 40 mm/h, elevated C-reactive protein (CRP) levels, hypoalbuminemia and age > 50 years have been proposed as clinical predictors17,24-29.
The treatment of patients with dermatomyositis and cancer is similar to that of cancer patients in general, provided that the cancer-dermatomyosits association does not imply any changes in the management of dermatomyositis. However, it has been reported that patients with dermatomyositis and malignancy tend to have a worse oncologic prognosis, since advanced stages are present at diagnosis and optimal resolution is not always possible17. Successful surgical management usually leads to complete resolution of dermatomyositis symptoms30,31. Early diagnosis of malignancy in these patients is paramount to achieve a better prognosis.
Most of dermatomyositis with colorectal cancer cases reported have affected women (63 %), and adenocarcinoma is the most frequent histological finding in dermatomyositis (96.3 %). In 77.7% of patients, dermatomyositis manifested before colorectal cancer with elevated CK levels. Immediate improvement of symptoms after surgery occurs in half of patients, in the remaining, improvement occurs within the first months after undergoing the surgical procedure31-33.
In the case reported here, dermatomyositis symptoms occurred a few months before the transverse colon carcinoma was found. Resolution of muscle symptoms and significant improvement of skin lesions took place several weeks after the lesion was surgically resected.
Conclusions
The exact cause of the association between dermatomyositis and the presence of malignancy is unknown. The presence of similar autoantigens in neoplastic, musculoskeletal and cutaneous cells has been suggested. Up to a quarter of dermatomyositis cases are associated, during the course of the disease, with the presence of cancer. At the time of diagnosis, patients with associated malignancy are already developing it, and generally the latter has a slow course of disease. When a patient is diagnosed with dermatomyositis, ruling out the presence of underlying malignancy, ideally in early stages, is of utmost importance to carry out interventions that positively contribute to the achievement of a better prognosis. Generally, after successful management (complete surgical resection) of the neoplastic lesion, dermatomyositis symptoms resolve; however, in case of tumor recurrence or metastatic cancer, they usually reappear.
The digestive tract is among the main sites of potential primary tumor origin, and within the digestive tract, stomach and colorectal cancer are the neoplasms most commonly associated with dermatomyositis. Finally, the evaluation of these patients must focus on their risk factors and associated clinical manifestations.











 text in
text in