Introduction
Despite the exponential growth of medical knowledge and technological and scientific progress in diagnostic methods and treatments, pancreatic diseases remain poorly understood, posing a diagnostic challenge. Symptoms are usually nonspecific or nonexistent (until advanced stages) because the retroperitoneal location of the pancreas makes its exploration difficult using traditional diagnostic imaging techniques. Considering that pathologies such as chronic pancreatitis, pancreatic cancer, and, more recently, fatty pancreas have been increasing in incidence and morbidity and mortality1,2, new diagnostic methods are required to diagnose them earlier and more accurately and provide timely treatment.
In 1980, DiMagno et al and Hisanaga et al developed endoscopic ultrasonography (EUS)3,4. Since then, it has become a diagnostic and therapeutic tool in pancreatic diseases, which better assesses the entire parenchyma, with reported sensitivities of 87-100%5,6. It has achieved better performance when compared to computerized axial tomography and magnetic resonance imaging6-8. Moreover, EUS plus fine-needle aspiration (EUS-FNA) has become the method to diagnose solid lesions in the pancreas, including pancreatic cancer, especially in lesions smaller than 30 mm, reaching a sensitivity of 93%6,9,10. However, up to 25% of patients may have false negatives, depending on various factors (type of needle, technique, experience, availability of a pathologist in the room, among others), added to its complications (such as pancreatitis and bleeding) and high cost (needles between USD 500 and 800), forcing the exploration of new cost-effective, non-invasive diagnostic methods with more outstanding performance. Therefore, EUS plus elastography has been explored as a complementary method.
Elastography evaluates tissue elasticity by ultrasonography, magnetic resonance, or optical coherence tomography11. It has shown its usefulness in the breasts12, prostate13, and thyroid14,15, as well as to guide or avoid biopsies of tissues, especially the liver. Although biopsy has been considered the gold standard for determining the fibrosis index, its use is limited by the invasiveness of the method (with significant morbidity and mortality), costs, and error in taking the sample, making it challenging to monitor the progression of fibrosis16.
Currently, transient elastography (FibroScanTM) has made it possible to establish the degree of fibrosis non-invasively, making it a widely used method16,17. It is less sensitive in patients with ascites, abundant adipose tissue, narrow intercostal spaces, and liver atrophy18,19 with a significant intra- and interobserver variability depending on the compression of the probe and less accuracy in the diagnosis of moderate fibrosis17,19,20. Therefore, real-time elastography (RTE) has emerged as the evolution of transient elastography, which may be more sensitive in diagnosing the degree of liver fibrosis. It will accurately determine that a liver fibrosis index (LFI) greater than 2.56 correlates with METAVIR scores of F421,22, even in the presence of inflammation23-27.
In the pancreas, RTE-EUS better characterizes different pathologies, such as chronic pancreatitis, fatty pancreas, and pancreatic cancer, by evaluating the rigidity of the pancreatic tissue with a color scale (qualitative elastography)28,29. In this field, pancreatic steatosis, an entity described in cadavers in 1926 and documented 40 years later concerning age and obesity30,31, has been classified as congenital and acquired. The latter is associated with obesity and metabolic syndrome, called pancreatic steatosis or nonalcoholic fatty pancreas disease32,33. The consequences are still under study; however, a relationship has been found with pancreatic cancer development, increasing its relevance 34,35.
Our group considers it could be used the same way but to assess the pancreatic parenchyma. In this pilot study, our objective is to assess whether the LFI measured by RTE-EUS is useful for differentiating normal, fatty, or cancerous pancreatic tissue in a cohort of patients evaluated using EUS.
Materials and methods
Study design and population
A prospective cross-sectional study was carried out at the University Hospital, a reference center for Gastroenterology and Ultrasound Endoscopy in Bogotá, Colombia, between January 2019 and January 2020. A total of 682 endoscopic ultrasounds were performed in patients who met the following criteria.
Inclusion criteria
Age equal to or older than 18 years (for both groups)
Patients referred for diagnostic ultrasound endoscopy without a history of the biliopancreatic disease (subepithelial or mucosal lesions, among others) (Group 1)
Patients sent for biopsy of solid pancreatic lesions, suspicious of cancer, whose cytology confirmed adenocarcinoma of the pancreas (Group 2)
Signed informed consent
Exclusion criteria
Patients with a history of acute or chronic pancreatitis
Patients who underwent an endoscopic ultrasound biopsy, but the pathology did not confirm the tumor
Patients with biliopancreatic disease other than cancer
Patients with portal hypertension of any etiology
Patients without a spleen
Patients with biliopancreatic anatomical alteration
Patients with decreased functionality greater than four on the Eastern Cooperative Oncology Group (ECOG)36 scale
Patients at risk of bleeding, with an international normalized ratio (INR) > 1.5 or with a platelet count < 50,000/mm2
Pregnant woman
Patients under 18 years of age
Patients who did not authorize the inclusion of their data in the study
Following the criteria, 66 patients were included for analysis (Figure 1).
Intervention
Patients who received endoscopic ultrasonography indicated by their treating physician, both inpatient and outpatient, and did not meet any of the exclusion criteria were questioned before the procedure, following a virtual form-type data collection tool. It gathered the demographic data, indication for the study, and the patient’s relevant history. A specialist in Internal Medicine and Gastroenterology fellow, previously trained to fill out the virtual form, simultaneously recorded the data obtained during the study. These data were downloaded, corrected, and entered into SPSS software (version 12.0; SPSS Inc).
In all patients, endoscopic ultrasonography was performed following the quality indicators of the American Society for Gastrointestinal Endoscopy and the American College of Gastroenterology37. They were carried out in the Gastroenterology ward of a reference unit for diagnostic endoscopic procedures under sedation guided by an anesthesiologist, with a combination of propofol and remifentanil, titrated according to each patient’s characteristics, after examination by the Anesthesiology service.
All procedures were performed with a Pentax linear echoendoscope (EG3870UTK; Pentax, Tokyo, Japan), combined with a portable Noblus color Doppler ultrasound system (Hitachi Aloka Medical, Tokyo, Japan), including the elastography module and another LFI module supplied by the manufacturer. An endoscopist experienced in interventional procedures, with more than 2,000 diagnostic and therapeutic EUSs, performed all EUSs.
The pancreatic parenchyma was evaluated during the examination, while the echogenicity was classified into Grades I to IV. The classification system was adapted from Sepe et al35. Besides echogenicity, the salt-and-pepper pattern of the pancreatic parenchyma and the clarity in delimiting the pancreatic duct were assessed. Grade I was defined as a pancreas in which more than 80% of the parenchyma is hypoechoic or isoechoic compared to the spleen, the major pancreatic duct is visibly outlined, and the salt-and-pepper pattern is observed. Grade II was defined as a pancreas in which more than 80% is hyperechoic compared to the spleen, the major pancreatic duct is visibly outlined, and the salt-and-pepper pattern is observed. Grade III was defined as a pancreas in which more than 80% is moderately hyperechoic when compared to the spleen, the major pancreatic duct is moderately obscured, and the salt-and-pepper pattern is moderately blurred. Grade IV was defined as a pancreas in which more than 80% is severely hyperechoic compared to the spleen, the margins of the main pancreatic duct are severely obscured, and the salt-and-pepper pattern is severely blurred. Grades I and II were considered normal, while grades III and IV as the fatty pancreas (Table 1)35.
Table 1 Fatty pancreas classification scale. Taken from35
| Grade | |
|---|---|
| I | Pancreas in which more than 80% of the parenchyma is hypoechoic or isoechoic compared to the spleen. The major pancreatic duct is visibly outlined, and the salt-and-pepper pattern is observed. |
| II | Pancreas in which more than 80% is hyperechoic compared to the spleen. The major pancreatic duct is visibly outlined, and the salt-and-pepper pattern is observed. |
| III | Pancreas in which more than 80% is moderately hyperechoic compared to the spleen. The major pancreatic duct is moderately obscured, and the salt-and-pepper pattern is moderately blurred. |
| IV | Pancreas in which more than 80% is severely hyperechoic compared to the spleen. The margins of the major pancreatic duct are severely obscured, and the salt-and-pepper pattern is severely blurred. |
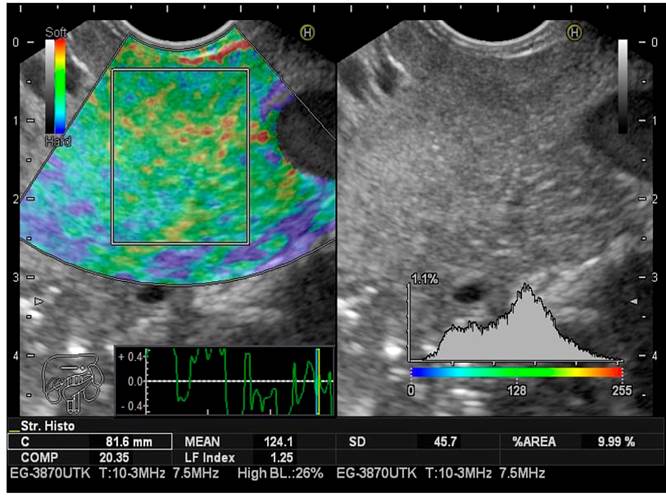
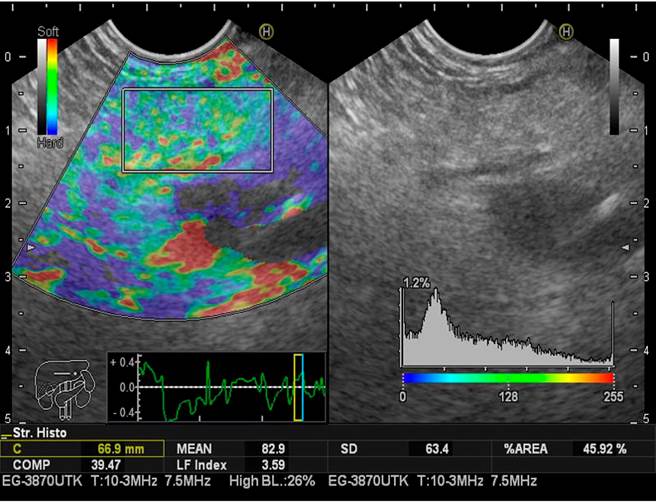
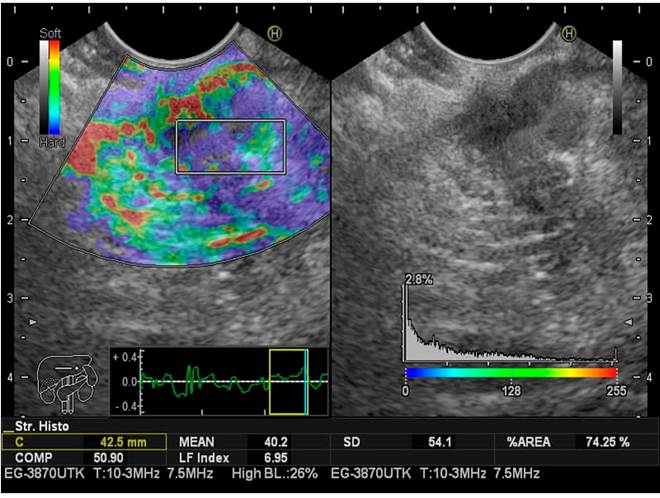
LFI taken for the pancreas was estimated three times during endoscopic ultrasonography in all patients to limit selection bias. The mean value of the three measurements was considered the final result of the analysis. As previously described, the LFI considers 11 parameters to find image patterns that assess the stiffness of the liver parenchyma. It is achieved by simply placing the cursor where we want to measure the LFI. The computer immediately calculates this index by pressing the “Enter” button. It has been used in abdominal ultrasound for the staging of liver fibrosis for approximately a decade using elastography images (RTE).
The sequential LFI is completed as follows:
Convert the selected analysis area of the RTE image to a 256-frame color scale image.
Plot the voltage histogram.
Calculate the mean of the relative stress (mean), the standard deviation of the relative stress (SD), the skewness of the strain histogram (SKEW), and the kurtosis of the strain histogram (KURT).
Binarize the RTE image into black and white regions: White as low voltage (blue) and black as all other regions.
To characterize the low-stress regions (blue), calculate the proportion of low-stress regions within the selected analysis area (area%) and the complexity of the low-stress region (COMP).
Calculate entropy (ENT).
Calculate the inverse difference moment (IDM) and angular second moment (ASM) to assess the texture of the RTE image
Perform multiple regression analysis to improve diagnostic accuracy using all of these imaging features, rather than diagnosing with individual imaging features, as shown in the following multiple regression equation23:
LFI = -0.009 x MEAN - 0.005 x SD + 0.023 x Area % + 0.025 x COMP + 0.775 x SKEW - 0.281 x KURT + 2.083 x ENT + 3.042 x IDM + 39.979 x ASM - 5.542
This calculation is done in milliseconds with the software, which does not significantly increase the time of the procedure (Figures 2,3y4).
Study variables and definition of variables
Table 2 shows the study variables and their definitions.
Table 2 Study variables and definitions
| Variable | Nature | Scale | Category |
|---|---|---|---|
| Age | Quantitative | Ratio Discrete | Years old |
| Sex | Qualitative |
|
|
| Background | Qualitative |
|
|
| EUS indication | Qualitative | Nominal Polychotomous |
|
| Classification of the fatty pancreas by ultrasound endoscopy | Qualitative | Nominal Polychotomous |
|
| Pancreatic fibrosis index | Quantitative | Continuous |
|
BMI: Muscle mass index; EUS: Endoscopic ultrasonography.
Statistical analysis
The database was registered in a virtual form (available on Google Drive with the shortened address shorturl.at/pIMWX). Then, this information was downloaded into Excel data tables, version 2013. We corrected, tabulated, and analyzed the data in the statistical package SPSS, version 2.1. Quantitative variables with normal distribution are presented as the mean and those with abnormal distribution as the median.
The univariate analysis was performed using a descriptive statistic to determine the absolute and relative frequencies in the qualitative variables. In the case of quantitative variables, central tendency, dispersion, or position measures were taken (using mean and standard deviation when the numerical variable follows the normal or median distribution).
We calculated the confidence interval for the data set based on the mean, standard deviation, and sample size for the data unit, with a P-value equal to 0.05. To determine the normal distribution of the numerical variables, the Kolmogorov-Smirnov statistic (KS statistic) was used, understanding that a p-value equal to 0.05 follows the normal distribution curve. An analysis of variance (one-way ANOVA) was performed to determine statistically significant differences between the means of these three groups (normal pancreas, fatty pancreas, pancreatic cancer), which is greater than 0.05. The prevalence will be expressed as a percentage. We sought to determine whether the LFI is different in the normal pancreas, fatty pancreas, and pancreatic carcinoma groups.
Results
In total, 66 patients were included. Group 1 included 55 patients sent for ultrasound endoscopy to assess pathologies other than a biliopancreatic disease; 32 were women, with an age range of 23-89, the mean age being 56.75 years. Group 2 had 11 patients diagnosed with pancreatic cancer confirmed by cytology, six women and five men, with an age range of 54-89, the mean being 69.56 years. The personal history of this population was high blood pressure, diabetes mellitus, obesity (defined as BMI greater than 30), hypothyroidism, and dyslipidemia, the most frequent being steatosis or steatohepatitis (n = 14), the mean being 25.45. The most frequent indication to perform the procedure was subepithelial lesion (n = 29). The percentages of patients according to the echogenicity of the pancreas were Grade I (n = 29), Grade II (n = 5), Grade III (n = 18); Grade IV (n = 3) (Table 3).
Table 3 Demographic characteristics of the study population
| Group 1 | Group 2 | |||
|---|---|---|---|---|
| Normal pancreas | Fatty pancreas | Total | Pancreatic cancer | |
| Age | n = average (range in years) 57.26 (25-72) | n = average (range in years) 55.90 (23-78) | n = average (range in years) 56.58 (23-78) | n = average (range in years) 69.56 (54-89) |
| Gender | Normal (n = 34); n = (%) | Fat (n = 21); n = (%) | Total (n = 55); n = (%) | Total (n = 11); n = (%) |
| Female | 23 (67.65) | 9 (42.86) | 32 (58.18) | 6 (54.55) |
| Male | 11 (32.35) | 12 (57.14) | 23 (41.82) | 5 (45.45) |
| History | ||||
| Steatosis or steatohepatitis (confirmed by a previous diagnostic imaging test, ultrasound, MRI, tomography) | 6 (17.65) | 8 (38.09) | 14 (25.45) | |
| Hypothyroidism | 5 (14.71) | 3 (14.29) | 8 (14.54) | 2 (18.18) |
| Diabetes mellitus | 4 (11.76) | 4 (19.05) | 8 (14.54) | 5 (45.45) |
| Dyslipidemia | 4 (11.76) | 3 (14.29) | 7 (12.73) | |
| Alcohol use (more than 14 g per week) | 2 (5.88) | 2 (9.52) | 4 (7.27) | 3 (27.27) |
| Arrhythmia | 1 (2.94) | 1 (1.82) | ||
| Coronary heart disease | 1 (2.94) | 1 (4.76) | 2 (3.64) | 1 (9.09) |
| Barrett’s esophagus | 1 (2.94) | 1 (1.82) | ||
| Dyspepsia | 1 (2.94) | 8 (14.54) | ||
| High blood pressure | 1 (2.94) | 2 (9.52) | 3 (5.45) | 5 (45.45) |
| Cerebrovascular disease | 1 (2.94) | 1 (4.76) | 1 (1.82) | |
| Kidney failure | 1 (2.94) | 1 (1.82) | ||
| No history | 6 (17.65) | 2 (9.52) | 8 (14.54) | |
| BMI classification. Taken from38 | ||||
| Underweight < 18.5 | 5 (14.71) | 1 (4.76) | 6 (10.91) | 5 (45.45) |
| Normal range (18.5-24.9) | 18 (52.94) | 5 (23.81) | 23 (41.82) | 6 (54.55) |
| Overweight (25.0-29.9) | 8 (23.53) | 10 (47.62) | 18 (32.73) | |
| Obesity (≥ 30) | 2 (5.88) | 4 (19.05) | 6 (10.91) | |
| Class I obesity (30.0-34.9) | 1 (2.94) | 3 (14.29) | 4 (7.27) | |
| Class II obesity (35.0-39.9) | 1 (2.94) | 1 (1.82) | ||
| Class III obesity (> 40) | 1 (4.76) | 1 (1.82) | ||
| Indication of the procedure | ||||
| Abdominal pain | 5 (14.71) | 3 (14.29) | 8 (14.54) | |
| Pancreatic tumor | 11 (32.35) | |||
| Gastric neuroendocrine tumor | 1 (4.76) | 1 (1.82) | ||
| Gastric extrinsic compression | 1 (2.94) | 1 (1.82) | ||
| Subepithelial lesion | 20 (58.82) | 9 (42.86) | 29 (52.73) | |
| Unexplained weight loss | 2 (5.88) | 2 (3.64) | ||
| Thick gastric folds | 3 (8.82) | 2 (9.52) | 5 (9.09) | |
| Gallbladder polyps | 1 (4.76) | 1 (1.82) | ||
| Elevated gastric lesion | 2 (5.88) | 4 (19.05) | 6 (10.91) | |
| Elevated duodenal lesion | 1 (2.94) | 1 (4.76) | 2 (3.64) | |
The LFI for the pancreas (mean value of the three measurements), taking Grades I and II as normal and Grades III and IV as fatty pancreas, were, respectively: Normal (n = 34), LFI 2.60, range 0.97-3.47; fatty pancreas (n = 21), LFI 3.87, range 2-5.5. There is a prevalence for the fatty pancreas of 38.18% when LFI is performed in three different subgroups considered endoscopically normal, fatty pancreas, patients with a previous diagnosis of pancreatic cancer confirmed by histology. The LFI taken in the pancreas was for the three groups, respectively: Normal (Grades I and II): LFI 2.60, range 0.97-3.47 (95% CI 2.17-3.02); fatty pancreas (Grades III and IV): LFI 3.87, range 2-5.5 (95% CI 3.44-4.29); pancreatic cancer: LFI 6.35, range 5.8-7.8 (95% CI 5.92-6.77) (Table 4).
Discussion
The fatty pancreas is a recently recognized condition that has been little investigated39. Different names have described these changes: Fatty pancreas, fatty infiltration, fatty replacement, and pancreatic lipomatosis. For this article, we have called it a fatty pancreas. Its spectrum ranges from the accumulation of interlobular fat (macrovesicles) to nonalcoholic pancreatic steatosis40. Thus, its epidemiology is not yet well defined, given the lack of parameters for a precise diagnosis and, as noted, the apparent differences in terminology to refer to the same entity.
A fatty pancreas is generally deemed an incidental finding during abdominal imaging for other reasons (with no standardized screening test yet). Epidemiological data are limited to the Asian population, and it is estimated that its prevalence may be between 16% and 35%41. A fatty pancreas has been associated with further development of pancreatic and metabolic complications. An association has been found with type 2 diabetes mellitus, acute pancreatitis (onset and severity), exocrine pancreatic insufficiency, chronic pancreatitis, pancreatic fibrosis, and even pancreatic carcinoma35,39-42.
Its pathogenesis is still under investigation, and data are scarce. However, an emerging hypothesis has been proposed that there is a more significant infiltration of macrophages in the pancreatic fatty tissue due to the alteration of the hormonal microenvironment, resulting in a chronic inflammatory condition characterized by the secretion of proinflammatory cytokines (TNF-α and IL-1β)42. Alternative hypotheses suggest that lipotoxicity resulting from triglyceride accumulation in β cells causes impaired glucose metabolism, leading to cell apoptosis and fat replacement. Paracrine signaling by adipocytes in the pancreas is added, which negatively regulates β cells. All of this alters glucose metabolism, which predisposes to type 2 diabetes mellitus41,42.
Pathways affected in acute pancreatitis involve increased production of free radicals, leading to injury and death of acinar cells. They can be replaced by fatty tissue, predisposing patients to develop more severe acute pancreatitis32-35,39-42. Finally, concerning pancreatic cancer, a fatty pancreas independently predisposes individuals to develop a pancreatic malignancy. Although the mechanism is unclear, this association may derive from chronic lipogenic inflammation while altering the microenvironment in a protumoral way32,34,42.
Assessment of the fatty pancreas includes a histological examination. The fatty infiltration of the pancreas bypasses the acini (exocrine function) and the islets of Langerhans (endocrine function) and preferentially accumulates within the pancreatic interstitial septa for unknown reasons. Usually, the entire pancreas is affected diffusely. Rarely, the ectopic fat deposition can be uneven, which occurs preferably in the head of the anterior pancreas, with preservation of the uncinate process, probably related to its embryological origin43.
The retroperitoneal location of the pancreas, the heterogeneous distribution of fat, and poorly defined borders make the fatty pancreas assessment challenging40-43; therefore, EUS plays a dominant role in this and other pancreatic diseases since it has significantly changed the diagnosis and management of 25% to 50% of cases44-48. However, an accurate diagnosis cannot always be determined using conventional EUS images alone. For example, in pancreatic cancer, the accuracy of EUS (without elastography) to differentiate cancer from benign lesions is approximately 75%39-43,49, requiring EUS-FNA, which reaches a sensitivity of 89%, specificity of 96%, and diagnostic accuracy of 97%37,50-52.
One of the difficulties in EUS-guided puncture is that tissue collection is technically demanding, and multiple punctures may be needed to obtain a sufficient amount of tissue47,48. Occasionally, despite repeated sampling, cytohistological evaluation may be falsely negative and may be associated with small but not insignificant morbidity rates53. Therefore, it is necessary to explore new methods to characterize lesions more precisely but non-invasively, limiting the need for biopsy in the areas with the highest suspicion of malignancy. One of these methods is EUS-guided elastography, which gave rise to the vibration technique in breast ultrasonography53,54.
Elastography is based on the knowledge that many pathological processes, such as fibrosis, inflammation, and cancer, induce alterations in tissue stiffness12,55,56. This technique assesses stiffness by applying slight compression. Using an ultrasound transducer on the target tissue and recording the displacement of the evaluated region, the physiological and respiratory vascular pulsations provide the vibrations (compressions) necessary for the study57-59.
There are different generations of initially qualitative elastography (colors), which served as the basis for the recently described second-generation elastography by EUS. It enables the quantitative evaluation of tissue stiffness with two different approaches: The strain ratio (SR) and the strain histogram (SH). This RTE-EUS can improve diagnostic performance non-invasively44-48,50-56,60-69.
The most studied of these methods is the SR by Itokawa et al, being a quantitative elastography technique to differentiate a lesion highly suggestive of cancer, with an average SR of 39.08 or more, from an inflammatory mass, with a mean SR of 23.66 or less (p < 0.05)61. Unfortunately, subsequent studies have not been consistent or reproducible, defining different cut-off values ranging from SR 3.7 to 24, with a sensitivity of 67-98% and with lower levels of specificity of 45-71%. Hence, its application in routine clinical practice is complex56,61,62,67. There is a need to assess other methods with more accurate and reproducible results. The answer could lie in the LFI, which, as explained previously, has already been validated, and its usefulness has been demonstrated in the non-invasive diagnosis of liver diseases, with an excellent correlation in measuring the stiffness of the liver parenchyma and detecting fibrosis and fatty liver23,25.
As explained above, the LFI is calculated automatically using software that uses the elastography images in real time; 11 variables are included. An initial finding of our work is that a third (38%) of the patients evaluated by EUS have a finding compatible with a fatty pancreas. Nonetheless, as reported in the literature, the prevalence in Asian countries and the United States ranges between 16% and 35%55,65. It may be related to the fact that 19% had obesity (BMI greater than 30) and 38% had fatty liver, documented by previous imaging. Of those with a fatty pancreas, more than 30% had some symptom of pancreatic insufficiency. From this study, it is not possible to establish relationships, even though a clear association has been found between nonalcoholic fatty pancreatic disease (NAFPD) with obesity and metabolic syndrome. Fatty degeneration and fibrosis in the pancreatic tissue surrounding most pancreatic adenocarcinomas have also been found33,70-72.
Therefore, detecting the fatty pancreas, especially the early detection of pancreatic cancer, is a permanent challenge in clinical practice. It is crucial to have an accurate tool for the early detection of these conditions, allowing timely medical intervention. Our proposal is to use the LFI measured by elastography through EUS.
We carried out this pilot study to evaluate its usefulness in the pancreas. This study found that, as in the liver, there is an association between the increase in LFI and tissue stiffness in the three groups of patients evaluated: Normal pancreas, fatty pancreas, and pancreatic carcinoma. We believe that it could be a useful and more objective tool than the SR if these results can be reproduced in studies with a larger and more heterogeneous population, making it possible to assess its performance in different scenarios of the fatty pancreas in controlled studies.
The main limitation is the sample size, as it is a single-center study with one observer. Being a pilot study with promising findings, it innovates in a field of medical knowledge still in development. It opens the door for new studies that clearly define the usefulness of this diagnostic and follow-up method in this and other settings.
Conclusion
This pilot study is the first in the world to use LFI applied to the pancreatic parenchyma. It was shown to help differentiate between normal and fatty pancreas and pancreatic carcinoma non-invasively; however, this finding must be validated in more extensive and heterogeneous populations.











 text in
text in