Introduction
Cirrhosis is the final phase of progressive and long-term liver disease1,2. The prevalence varies in each country, with a maximum incidence between 40 and 50 years, affecting more men2. In Western countries, 90% of cases are due to alcohol abuse, non-alcoholic fatty liver disease (NAFLD), and chronic viral hepatitis, including hepatitis B (HBV) and hepatitis C (HCV). About 10% of the etiology of this entity is unknown1-3.
Cirrhosis is the consequence of the continuous death of hepatocytes, with loss of parenchyma, inflammation, fibrogenesis, changes in cell regeneration, and alterations in macro- and microcirculation4-6. It is a dynamic and reversible process at some point7-9, which has motivated studies in the search for adequate management by identifying and monitoring asymptomatic patients and preventing the entity’s complications. Four of them are classic decompensations: Ascites, variceal bleeding, encephalopathy, and jaundice10-12.
The manifestation of any of them markedly decreases the survival of cirrhotic patients, being worse with hepatocarcinoma (HCC)12-15, which has an approximate annual incidence of 7%1,12,15 and is associated with the same causes of cirrhosis, HCV, alcohol, and NAFLD1,16-20. Fatty liver is a cause of cirrhosis on the rise21,22, directly correlated with elements of the metabolic syndrome. For example, type 2 diabetes mellitus, obesity, and high blood pressure, all with increased cardiovascular risk23. Therefore, this study aims to compare a cohort of patients diagnosed with cirrhosis, evaluate their complications and survival according to their etiology, describe clinical and laboratory aspects, and determine the role of fatty liver.
Materials and methods
We carried out a retrospective cohort study. It included patients who attended a specialized Hepatology consultation at the Center for Liver and Digestive Diseases (CEHYD) in Bogotá between January 2010 and June 2019. Confirmed diagnosis of cirrhosis by the clinic, radiology or liver biopsy, and available medical records were the only inclusion criteria.
Based on the Child-Pugh (CP)24 cirrhosis severity scale, we defined:
Compensated cirrhosis: A Child A stage, without any additional decompensation
Decompensated cirrhosis: A Child B or C stage, or a Child A cirrhosis with ascites, variceal bleeding, encephalopathy, or jaundice
Additionally, ascites, variceal bleeding, encephalopathy, jaundice, HCC, hepatorenal syndrome, or coagulopathy were defined as complications of cirrhosis.
The information collected was summarized using means and standard deviations for a normal distribution (Shapiro-Wilks test); otherwise, it was summarized with medians and interquartile ranges. We performed the analysis with non-parametric statistics and summarized categorical variables as proportions. Survival was assessed based on medians and interquartile ranges. We used the Kaplan Meier estimator of the survival function and the log-rank test. A Cox hazard model was made for the analysis adjusted by the effect of confounding covariates. STATA v15.1 and R were employed for statistical analysis.
Ethical considerations
As a retrospective study, no intentional intervention or modification of the individuals’ biological, physiological, psychological, or social variables was carried out. It was conducted under the principles declared at the 18th WMA General Assembly (Helsinki, 1964) as a world reference for research on human beings. As set forth in Article 11(a), Title II (about research on human beings), Chapter I (about the ethical aspects of research on human beings) of Resolution 008430 dated October 4, 1993, issued by the Ministry of Health of the Republic of Colombia, this study constitutes research without risk. It uses retrospective documentary research techniques and methods, including medical records, interviews, questionnaires, and others, in which sensitive data on illnesses are not identified or processed.
Since it has no risk and only uses documentary research techniques and methods, this study is understood to follow the fundamental principles of ethics: Beneficence, autonomy, justice, and non-maleficence.
Results
We reviewed a total of 1,200 medical records, of which 681 (56.8%) were women. The mean age at diagnosis of cirrhosis was 63 years (interquartile range [IQR] 56-71). The death occurred in 51 patients, with a median age of 75 years (IQR 65-80). The patients were monitored over time, measured in months, with a median of 17.4 (IQR 5.5-45.3). The first complication of cirrhosis occurred in 545 patients (45.4%) 0.7 months after diagnosis (IQR 0-18.1). Complications were, in order of importance, ascites (33.8%), variceal bleeding (22.2%), HCC (17.4%), jaundice (14.3%), encephalopathy (7.3%), ascites plus encephalopathy (2.6%), coagulopathy (2%), and hepatorenal syndrome (0.4%). The clinical and demographic characteristics are shown in Table 1.
Table 1 Clinical and laboratory variables in patients with cirrhosis
| Variable | Number | Median (IQR)* |
|---|---|---|
| BMI kg/m2 | 1139 | 27 (24 to 30) |
| White cells (cell/mL) | 1154 | 5500 (4400 to 6890) |
| Hemoglobin (g/dL) | 1153 | 14 (13 to 16) |
| Hematocrit % | 1153 | 43 (38 to 47) |
| Platelets (cell/mm3) | 1155 | 158 000 (111 650 to 224 500) |
| Glycemia mg/dL | 1087 | 98 (88 to 115) |
| AST IU/dL | 1158 | 54 (34 to 90) |
| ALT IU/dL | 1161 | 50 (31 to 87) |
| GGT IU/dL | 1049 | 120 (56 to 259) |
| Alkaline phosphatase IU/dL | 1125 | 132 (95 to 211) |
| Total bilirubin (mg/dL) | 1143 | 1 (0,6 to 1,7) |
| Direct bilirubin (mg/dL) | 1139 | 0,4 (0,2 to 0,8) |
| Indirect bilirubin (mg/dL) | 1137 | 0,5 (0,3 to 0,9) |
| Total protein (g/dL) | 1044 | 7,3 (6,9 to 7,8) |
| Albumin (g/dL) | 1100 | 4 (3,4 to 4,3) |
| INR | 1117 | 1,1 (1 to 1,2) |
BMI: Body mass index; INR: International normalized ratio; IQR: Interquartile range tested for normality (Shapiro-Wilk test).
According to the cirrhosis etiology, the patients were classified into five groups (Table 2). The lowest median age at diagnosis of cirrhosis was for the cholestatic group. Furthermore, the alcohol group had a lower median age at death, 64 years. The etiological group with the highest percentage of complications was again alcohol (63.5%), and the lowest was the non-alcoholic steatohepatitis (NASH) group, with 31.6%.
Table 2 Differences between groups by etiology
*Significant differences (p < 0.005) between etiological groups of cirrhosis, Fisher’s exact test.
**Significant differences (p < 0.005) between etiological groups of cirrhosis, Kruskal-Wallis test.
IQR: Interquartile range.
Regarding the severity of cirrhosis at admission, 69.6% had Child A (higher in the NASH group), 21.3% Child B, and 3.9% Child C (higher in the alcohol group).
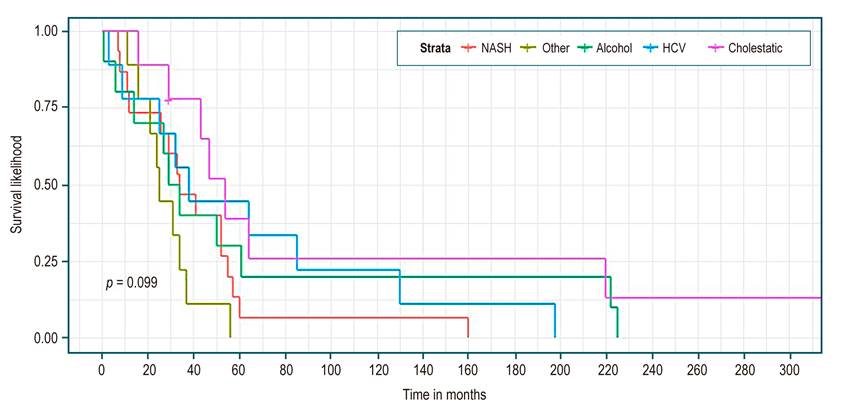
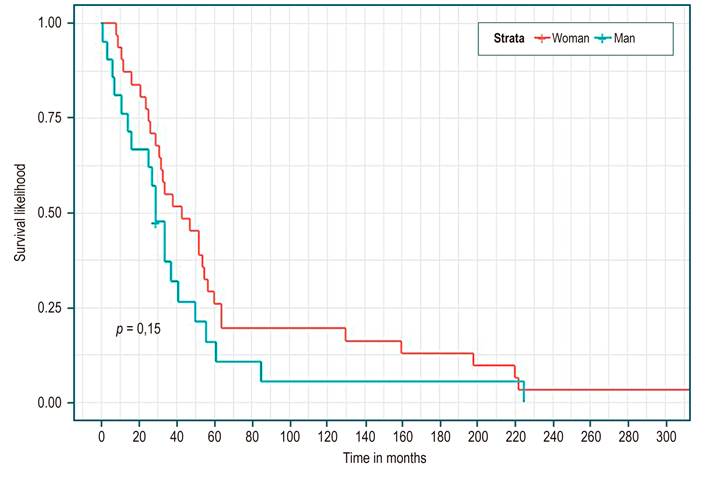
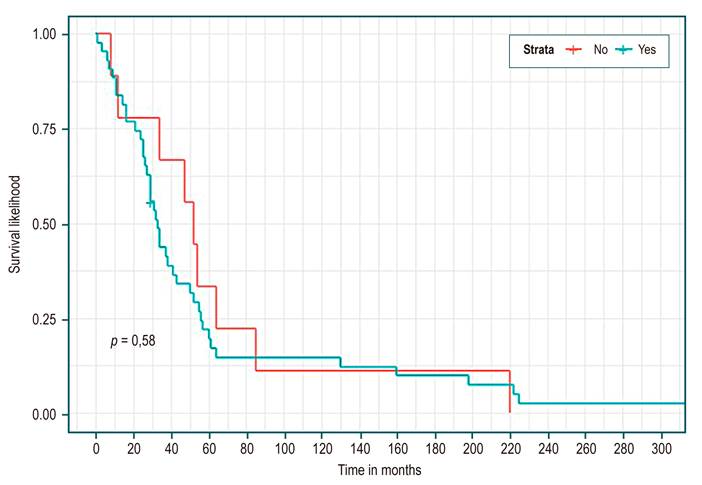
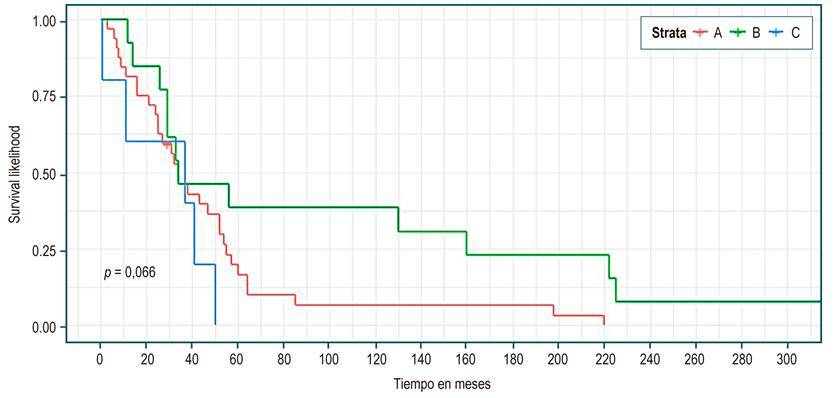
Table 3 shows, in all patients, a median survival of 34 months (95% CI 29-52). There were no statistically significant differences in median survival between the groups of etiology, sex, presence or absence of complications, or Child. However, higher survival was identified in the cholestatic group (Figure 1), being a woman (Figure 2), not having a complication (Figure 3), and Child C (Figure 4), the median survival being 54, 43, 52, and 37 months, respectively.
Table 3 Survival by group and difference comparison
| Number of events | Median survival (95% CI) (months) | Log-rank test | |
|---|---|---|---|
| Total | 51 | 34 (29 to 52) | |
| Etiology of cirrhosis (Figure 1) | |||
|
|
|
|
p = 0.099 |
| Gender (Figure 2) | |||
|
|
|
|
p = 0.15 |
| Complications (Figure 3) | |||
|
|
|
|
p = 0.58 |
| Child* (Figure 4) | |||
|
|
|
|
p = 0.066 |
NC: Not calculated.
*In two patients, it was not calculated due to a lack of data
Considering the variables and the follow-up time illustrated in Table 4, the univariate analysis found that as the age at diagnosis of cirrhosis increases, the risk of death is higher; HR 1.04 (95% CI 1.02-1.075; p = 0.000633). For each month that the follow-up of cirrhotic patients increases, the risk of death is reduced by 90%; HR 0.1 (95% CI 0.03-0.29; p < 0.00). Similarly, a longer follow-up of complications reduces the risk of death by 2%; HR 0.98 (95% CI 0.97-0.99; p = 0.000695). Finally, the group of other causes of cirrhosis presents a risk of death 3.87 times compared to the cause of cirrhosis due to cholestatic disease (p = 0.0386), statistically significant estimates.
Table 4 Differences between cirrhosis groups and follow-up
| Follow-up variables | HR (95% CI) (univariate) | |
|---|---|---|
| Age at first consultation in years | 1.02 (0.99 to 1.04) | p = 0.096 |
| Age of diagnosis of cirrhosis in years | 1.04 (1.02 to 1.075) | p = 0.000633 |
| Follow-up of cirrhosis from its diagnosis in months | 0.1 (0.03 to 0.29) | p <0.00 |
| Follow-up of complications after the diagnosis of cirrhosis in months | 0.98 (0.97 to 0.99) | p = 0.000695 |
| Etiology of cirrhosis | ||
|
|
|
|
| Sex | p = 0,156 | |
|
|
|
|
| Complications | p = 0,591 | |
|
|
|
|
| Child | ||
|
|
|
|
Discussion
Studies in the United States showed that in 2010, chronic liver diseases and cirrhosis were the cause of 31,903 deaths, with age-adjusted mortality of 9.4 per 100,000 individuals, and decompensated cirrhosis accounted for more than 150,000 hospitalizations, with a cost close to 4 billion US dollars25,26. These data highlight the importance of monitoring the cirrhotic patient.
When reviewing this cohort, the average age of cirrhosis was 63 years, as reported in the Swedish population study by Nilsson et al26, with a follow-up of more than 1,000 patients. The highest incidence of the disease occurred in the group of 60-64 years, with a predominance in the female sex, as in our series. In other international series, the male gender predominates, probably due to etiological differences27,28.
D’Amico emphasizes a different prognosis in terms of mortality according to the presence or absence of decompensations12,28,29. In our cohort, the analysis focused on the presence or absence of complications. Classic ascites, variceal bleeding, jaundice, and encephalopathy12 were added to HCC, hepatorenal syndrome, and coagulopathy. In this series, complications occurred in 45.9% of all patients shortly after the diagnosis of cirrhosis (0.7 months) (IQR 0-18.1) and with a total follow-up of 17.4 months (IQR 5.5-45.3). European series show follow-up periods of up to 10 years or more27,28. These two pieces of data alert us to the lateness of our diagnoses since, in most cases, the complication leads to the diagnosis of cirrhosis, and advanced disease does not allow for further follow-up.
In different studies, the survival of patients with compensated cirrhosis is relatively good, with a median of 12 +/- 2 years on average30-32. Our data in patients without complications show a median survival of four years and four months (52 months), well below international data. Although it is higher than the group with complications, it does not present statistically significant differences and could be explained by the number of events analyzed. Moreover, the mortality rate (HR) in patients with complications was 1.22 (CI 0.58-2.53; p not significant), similar to Nilson’s study after the first year (HR 1 .44, CI 1.23-1.68)26. Again, these results suggest that our population probably requires more medical follow-up for their cirrhosis.
According to Child (Table 3), the results are similar when analyzing survival between 34 and 37 months for the three groups. Although it seemed to be longer for Child C, it favored Child B in the survival curve (Figure 4). No statistically significant differences were found in any case. These findings are explained by the low number of events (n = 51); however, it is expected that this cohort of patients will be monitored to clarify these results.
Although the survival data by cirrhosis etiology (Table 3 and Figure 1) did not present statistically significant differences, they seemed to favor cholestatic disease (HR reference pattern in Table 4). Apparently, it is an etiological cause of cirrhosis with a better prognosis, probably due to its onset in childhood or younger populations, with earlier diagnosis, better follow-up, and, in theory, less liver damage31. In a study with 9,261 patients, the cholestatic disease had a mortality rate per 100 patients/year of 6.3 (3.1-12.5) below alcohol, virus, and NASH, this being the highest at 15.2 (12.9-17.8)32. These trends are similar to our study, with HR for alcohol, HCV, and NASH of 1.56, 1.62, and 2.36, respectively (Table 4).
Having other causes of cirrhosis increases the risk of death 3.87 times (Table 4). This group comprises etiological combinations that result in more progressive liver damage. European HCV studies21-23 mention that having multiple risk factors, such as viral factors, fatty liver, and alcohol, increases the likelihood of death, as suggested in other studies12,15,27.
Our data show NASH as the primary etiology of cirrhosis (33.2%). The importance of fatty liver as a cause of liver disease and cirrhosis is corroborated by local and international series, where it is shown to displace other etiologies11,19,33-37). It must be remembered that worldwide, between 20% and 40% of the population suffers from it. A meta-analysis involving 8,515,431 estimated this global prevalence at 25%, with prevalence rates in South America of 31%38. Therefore, NASH as a cause of cirrhosis and its complications could even be underdiagnosed.
Despite being a single-center study, this is a Colombian cohort of patients under follow-up. It provides research options to learn about our reality and evidence of the fatty liver pandemic and its association with cirrhosis.
Conclusions
In the present cohort of cirrhotic patients, survival by etiology, gender, or presence/absence of complications did not show statistically significant differences; however, these complications manifest very quickly and alert us to late diagnoses. NASH was the leading cause of cirrhosis, and efforts should be directed towards its diagnosis and management in the early stages.











 text in
text in