Introduction
Colorectal cancer is the third most common type of cancer in Colombia. Nineteen thousand two hundred fifty-eight new cases were diagnosed in 2018. Only 13% of them corresponded to stage I, while 40% were in stages III or IV. 26% of the cases did not have a correct staging1. The opportunity for diagnosis and management has improved, but there are still many patients without adequate oncology treatment. Rectal cancer is a complex disease. Its own natural history and difficult anatomical location make imaging evaluation less accurate, and the surgical dissection is more demanding. These attributes produce substaging, inappropriate neoadjuvant and adjuvant treatments, and surgeries with resection margins that, if positive, will decrease the probability of healing and long-term survival with an adequate quality of life.
This is why it is pertinent to review the recent changes in evaluation images, staging, surgical technique, and complementary treatments. Those that provide better results for the patient will be chosen and implemented in clinical practice routinely.
Changes in oncology nomenclature
The new terminology emerges from the new tools in imaging studies and the new anatomical concepts. It must be standardized and managed by all the specialists involved in management2. Note especially the different staging possibilities according to the magnetic resonance imaging (MRI) during the preoperative. Table 1 describes the current terms and their abbreviations used in this review.
Table 1 Oncology nomenclature
| CRM: Circumferential resection margin |
| cTNM: Clinical staging according to AJCC |
| EMVI: Extramural vascular invasion |
| MRF: Mesorectal fascia |
| MSI: Microsatellite instability |
| mrTNM: MR staging |
| pTNM: Definitive pathological staging |
| CR: Complete response |
| LR: Local recurrence. Tumor reappearance within 5 years of pelvic follow-up |
| OS: Overall survival. Live patients after 5 years of follow-up |
| PFS: Progression-free survival |
| TME: Total mesorectal excision |
| TNT: Total neoadjuvant therapy |
| ypTNM: Post neoadjuvant definitive pathological staging |
| yTNM: Imaging staging after neoadjuvant therapy |
AJCC: American Joint Committee on Cancer; MRI: Magnetic resonance imaging. Source: Table made by authors
Changes to clinical guidance guidelines
Cancer reference guides have had changes in their updates. There are two main guides: The National Comprehensive Cancer Network (NCCN)3 and the European Society for Medical Oncology (ESMO)4. When comparing, there are fundamental differences in both. ESMO guidelines have had greater adherence in surgeons recently. However, most oncology groups follow the guidelines defined by NCCN.
Differences between recommendations in NCCN and ESMO guidelines
For years, in the NCCN guidelines (Figure 1), the preoperative study was based on rectal ultrasonography (2004-2012), defined by Sauer’s study, where ultrasonography was indicated in all patients5. In the 2017 update, MRI was suggested as the preferred study, and in May 2020 update, simple rectal MRI became the study of choice. Rectal ultrasonography is only for early selective cases. In this last update, CRM was considered a fundamental factor in decision-making to define surgical management, indication, and type of neoadjuvant, and as a determinator of different chemotherapy schemes2. Decision-making continues to be based on classic TNM, where un-subclassified T3, T4, and positive nodes are the primary indication for neoadjuvant chemoradiotherapy (CRT).
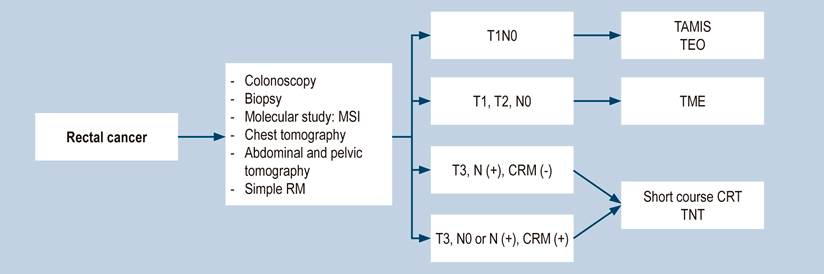
Figure 1 Recommendations for the management of rectal cancer according to NCCN guidelines. CRM: Circumferential resection margin; MSI: Microsatellite instability; N: Nodal state; CRT: Chemoradiotherapy; MRI: Magnetic resonance imaging; T: Primary tumor size, TAMIS: Transanal minimally invasive surgery; TEO: Transanal endoscopic operation; TME: Total mesorectal excision; TNT: Total neoadjuvant therapy. Taken from3.
The European ESMO guidelines (Figure 2) changed the parameters of decision-making based on the Mercury6) studies. Primary tumor size (T) is subclassified according to anterior or posterior mesorectal fat penetration at T3a (< 1 mm), T3b (2-5 mm), T3c (> 5 mm), and T3d (> 15 mm). Positive or negative CRM determines decision making, i.e., nodal status is important but not paramount. Staging is based on the subclassification of T3 and the status of CRM6,7. According to these guidelines, patients with T1, T2, and T3a and b CRM (-) can be directly taken to surgery.
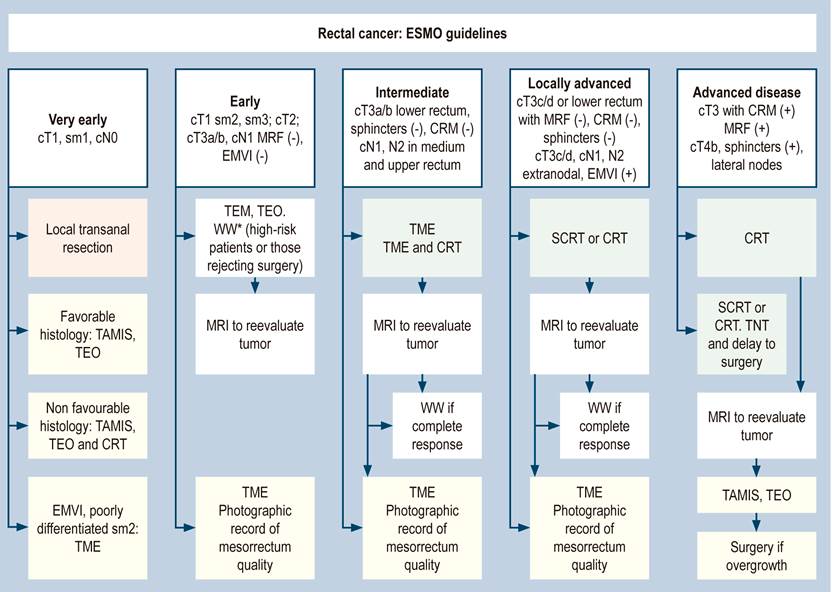
Figure 2 Recommendations for rectal cancer management according to ESMO. cN: Clinical nodal status; CRM: Circumferential resection margin; cT: Primary cynical tumor size; EMVI: Extramural vascular invasion; MRF: Mesorectal fascia; CRT: Chemoradiotherapy; MRI: magnetic resonance imaging; sm: submucosa; TAMIS: Transanal minimally invasive surgery; TEM: Transanal endoscopic microsurgery; TME: Total mesorectal excision; TEO: Transanal endoscopic operation; TNT: Total neoadjuvant therapy; WW: Watch and wait. Source: ESMO rectal cancer guidelines.
In the ESMO guidelines, neoadjuvant therapy is recommended in T3c, T3d, T4, and CRM positive or at commitment risk. A good surgical technique ensures the resection of all nodes under TME, which was defined and standardized by Heald in 1986. Positive nodes are not an absolute factor in making decisions before operating8. Positive nodal involvement is important in neoadjuvant decision-making if it is part of a positive CRM. If the patient has positive nodes in the MRI, but the MRF and CRM are free, this does not necessarily imply sending the patient to CRT. This is a fundamental difference in the ESMO guide.
The ESMO and NCCN guidelines consider organ preservation strategies, in which patients with tumors classified as cT2N0M0 or cT3a and b of the lower rectum may be included for neoadjuvant therapy. The ESMO guide recognizes the “wait and watch” (WW) protocol, reported in Brazil more than 14 years ago by Habr-Gama et al, not only as a protocol followed in experimental groups8 but as a reality to follow, where a complete clinical response is obtained in 37% to 50% of patients9,10.
Changes in anatomical diagnosis
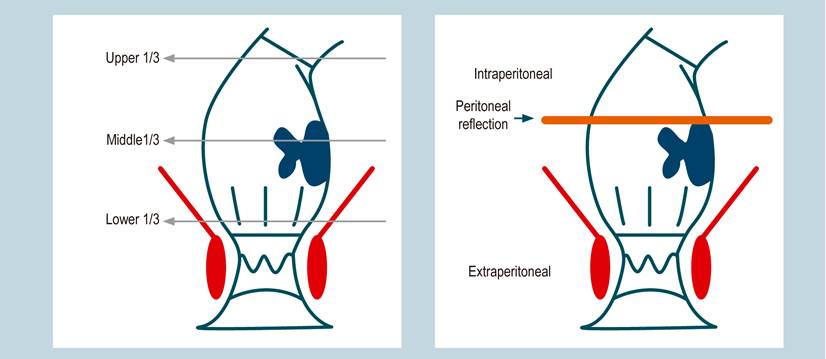
An anatomical diagnosis must be performed for rectum cancer. The diagnosis has been traditionally defined by the third portion, where the tumor is located. The rectum is divided into the upper, middle, and lower third. The lower third ranges from 0 to 5 cm, the middle from 5 to 10 cm, and the upper one from 10 to 15 cm from the anal ridge. It is important to describe if the tumor is felt by digital rectal exam and if it is fixed. Semiologically, the distance in centimeters may vary when the colonoscope, which is flexible, is taken as a measuring instrument. The use of rigid rectosigmoidoscopy to measure this distance has been described, but this is a recommendation with little adherence. It is advisable, as a tool, not only to define the tumor height in centimeters and its relationship with the Houston’s rectal valves during colonoscopy. Also, it is recommended to establish the location of the peritoneal reflection using MRI. It simply defines whether the tumor is intraperitoneal or extraperitoneal and whether the peritoneal reflection is compromised.
The lower third will comprise the extraperitoneal rectum, where the CRM is evaluated. The anterior face in the middle third is intraperitoneal, and its lateral and posterior faces are extraperitoneal. The MRF will be evaluated in this area, while the upper third is intraperitoneal. The distance from the anal ridge to the tumor in centimeters, the location and fixation of the tumor by a digital rectal exam, the colonoscopic description, and the MRI with the peritoneal reflection assessment will determine the anatomical diagnosis (Figure 3)11. However, tumors in the middle third could have an intraperitoneal and extraperitoneal component. This particular aspect will be definitive to determine the neoadjuvant decision and the surgical technique to be performed: a total or partial excision of the mesorectum.
Changes in pharmacological management by the oncologist
Management is determined by the degree of tumor tissue invasion (T3, T4), the number of nodes, and negative section edges. Finding more than 12 nodes was the quality measure for surgery in rectal cancer. Less than 12 nodes were considered an incomplete surgery, a candidate for adjuvant therapy. Currently, the oncologist must analyze the same factors as the radiologist, the surgeon, and the pathologist (the CRM, the TME quality, and the presence of EMVI). From the point of view of LR and OS, these additional factors are the ones that have the most impact. The most interesting thing is that they can be evaluated with a simple MRI without contrast from the beginning of the patient’s study, in the follow-up MRI after neoadjuvant surgery, and confirmed in the definitive pathology12.
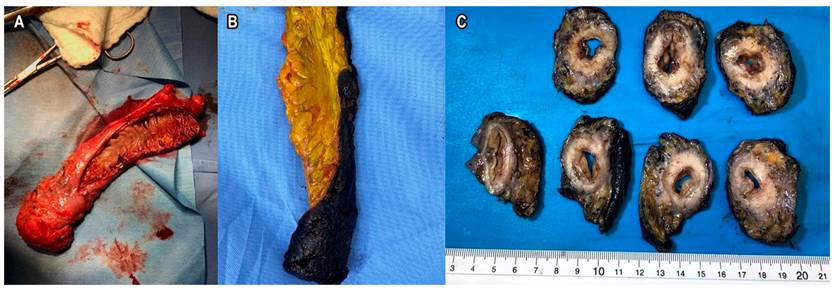
The positive resection margin, or rectal circumferential (both definitions are valid), is the presence of tumor, node, or EMVI less than 1 mm from the circumferential margin in the extraperitoneal lower rectum. That edge is determined when reviewing the MRF in the MRI or the Indian ink in the pathology. If the measurement is in the upper rectum, it will correspond to MRF. If the measurement is in the extraperitoneal rectum, it will correspond to CRM. Of these factors, the one with prognostic value and affects the LR and OS is the positive CRM given by tumors (Figure 4)13,14.
Changes in staging images
Simple MRI with rectal protocol is the primary examination for staging and defining the subclassification of T, CRM, and the presence of EMVI. The inferior rectum provides other parameters, such as the tumor relation with the sphincterial external mechanism and the intersphinteric groove. Properly reporting the CRM involves the perfect description of the MRF. Recognizing it in the images ultimately helps establish an adequate staging, make the oncological management decisions, and plan the surgical technique.
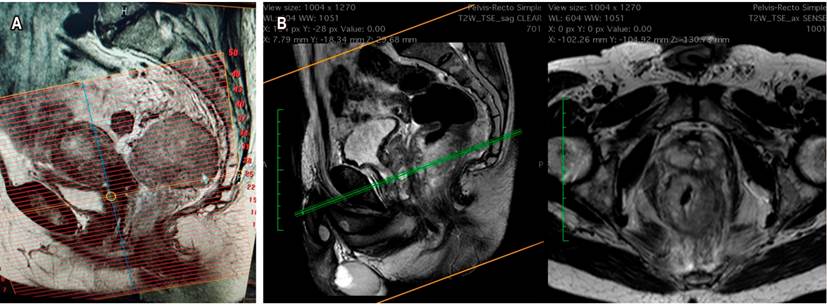
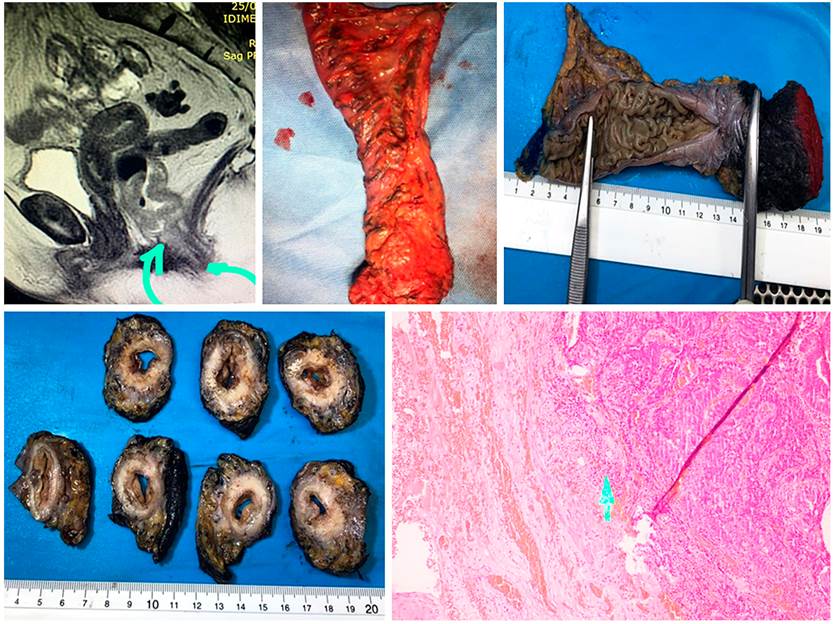
If the neoadjuvant decision was made by positive CRM or by the risk of being positive, restaging is done with an additional simple MRI, evaluation of T2 sequences, restriction to diffusion, and tumor regression index. This evaluation will be done depending on the type of neoadjuvant therapy performed, if conventional, 6 weeks after the radiotherapy is finished. If it were TNT (a new concept), the measurement would be made at the end of the consolidation chemotherapy. One of the frequently found difficulties in the study is that it is usually requested as a pelvic MRI. If the MRI with rectum protocol is not specified and requested, the technician will make the alignment and reconstruction of the images in an inadequate way. These images will not be perpendicular to the rectum and the tumor axis, and it will not be possible to evaluate the subclassification of the T correctly. It is advisable to establish, with their working group, the revision of the image reconstruction technique (Figure 5)14,15.
Changes related to the indication of surgery as initial treatment
Patients with preoperative staging reporting T1N0M0 would be candidates for TME or transanal minimally invasive surgery by any of the existing platforms: transanal minimally invasive surgery (TAMIS), transanal endoscopic operation (TEO), or transanal endoscopic microsurgery (TEM).
Lower third T2N0M0 patients may be taken to initial surgery or be candidates for an organ preservation protocol. This decision should arise at a multidisciplinary board as part of a neoadjuvant CRT plus consolidation chemotherapy program (this is the WW protocol). All patients with upper third cancer are candidates for immediate surgery, as long as the MRI does not describe peritoneal reflection involvement or risk of MRF involvement. Likewise, patients with lower, middle, or upper third cancer classified as T2 or T3a/b, negative CRM, with intact MRF, and whom the surgeon believes is feasible to perform a TME or partial resection of the mesorectum with 5 cm of distal margin, can go to surgery directly.
Neoadjuvant treatment is reserved for T3c/d and T4 patients, those with tumors with positive CRM or at involvement risk of the circumferential margin and with extension to the intersphincteric groove, as well as patients with selected N1, N2, or lateral nodes. This has represented a fundamental change in the management algorithm. The nodal status is not necessarily what determines the need for neoadjuvant therapy. According to the ESMO guidelines, the presence of positive nodes in the middle or upper third, with free MRF, can be taken to surgery. This is a big difference from the NCCN guidelines’ recommendations, which send patients to chemotherapy and radiotherapy first. Patients with lower rectal cancer, where their surgery may involve performing an abdominoperineal resection (ARP), or an intersphincteric coloanal resection, may be treated with an organ preservation strategy from T2N0 onwards. New radiation schemes and consolidation chemotherapy may generate a higher percentage of complete or near-complete clinical responses with this strategy.
Neoadjuvant therapy allows a complete clinical response in the organ preservation strategy, which is the basis of WW. However, in certain cases, it could give an almost complete response or a decrease in staging in T, a scenario that can be managed with local resection, a TAMIS or TEO approach, and that should only be performed by expert groups in the management of rectal cancer16,17. This last approach in the almost complete response after neoadjuvant therapy is still under discussion.
The complete clinical response we observed in our setting is between 11% and 20% when using standard CRT. The schemes used in Brazil are extended CRT with 5400 centigray (cGY) and consolidation chemotherapy based on 5-fluoracil or oral capecitabine18. This scheme is indicated in patients with preoperative staging from T2N0M0 onwards, who obtain a 50% CR. Complete clinical response after TNT has been reported in 50%-65% of patients in certain studies, such as the OPRA19 study. These results were not seen with previous algorithms. Other reports indicate an overall rate of 37% CR when patients with early and locally advanced stages are included.
Changes in the neoadjuvant therapy
Neoadjuvant therapy is indicated in patients with upper third rectal cancer with peritoneal reflection involvement, patients with T3c/d middle and lower third rectal cancer, positive T4, CRM, and EMVI, and in organ preservation strategy in the lower rectum in patients ≥ T2N0M020.
There are several definitions that should be clarified in chemotherapy. As part of standard CRT, chemotherapy is called 5-fluoracil or capecitabine-based sensitization chemotherapy. If a complete scheme is applied before radiotherapy, it is called induction chemotherapy. If it is applied concomitantly or after standard CRT, it is called consolidation chemotherapy. Consolidation chemotherapy with 5-fluoracil or capecitabine is used in the WW protocol. The 16-week oxaliplatin addition scheme is usually applied when staging includes positive n and CRM, or T4 additionally. TNT groups together all the schemes where systemic chemotherapy, which used to be given after surgery, is now given before surgery.
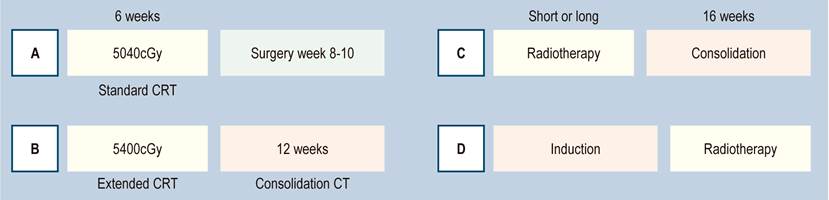
Radiotherapy schemes may be a short course, where the patient receives 500 cGY for 5 days, for a total of 2500 cGY. Waiting 4 to 6 weeks produces the same biological effect as the conventional long scheme, as long as 6 to 8 weeks are taken to wait to perform the surgery. If used as part of TNT, the patient receives consolidation therapy 15 days after the end of the short cycle. The long standard radiotherapy scheme is 5040 cGY in 25 sessions of 200 cGY per day, applied in 6 weeks. The Brazilian scheme applies 5400 cGY, corresponding to 4500 cGY of external radiotherapy and an additional reinforcement (boost) of 900 cGY focused on the tumor. In practical terms, it is 2 more days of radiotherapy, followed by consolidation chemotherapy (Figure 6)18.
Changes at the time of operating
T1 tumors are treated with TME or local transanal resection. T2, T3a/b lesions of the middle and lower third should go to surgery only if a TME is feasible. If the lesion is in the lower rectum, and an organ preservation scheme is considered, extended CRT plus consolidation is given. At the end of chemotherapy, it is re-evaluated, and, based on the results, the decision to operate or to observe if a complete clinical response was obtained is made. If the answer is almost complete (a new concept to be discussed), the observation can be extended to 6 weeks to achieve a CR and make an organ salvage21.
Combining the classics and new concepts for a T3c/d, middle and lower third T4, we have the following alternatives:
Traditional scheme: Long-course CRT. Wait for 6 weeks and re-evaluate with MRI. The surgery is performed between weeks 8 to 12. After this, it is decided if there is adjuvant chemotherapy. This is the most used scheme of neoadjuvant therapy, surgery, and adjuvant care, which we are already abandoning.
Before a tumor with a high risk of sphincter loss, it is possible to choose a TNT scheme with 4-month consolidation chemotherapy, re-evaluation with MRI, and surgery performed at 6 months. The TNT schedule can be performed with short or long scheme radiotherapy and induction or consolidation chemotherapy. In the case of CR, the WW protocol can be applied to save the rectum. If the response is almost complete, you can opt for a TME or a minimally invasive transanal approach to achieve the preservation goal.
Some studies support each of these options and others are in progress to define the best strategy with impact on LR and OS (RAPIDO and OPRA studies). Preliminary reports give complete clinical responses between 50% and 64% of cases(22.23).
What changed in the surgery?
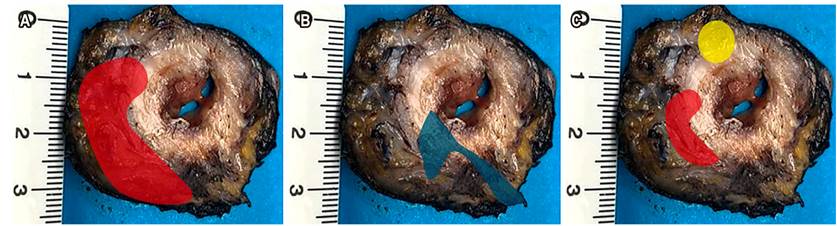
Surgery is no longer defined in terms of anterior resection, low anterior resection, or ultra-low anterior resection. Currently, it is described in terms of TME for lower third tumors, some middle-third tumors with extraperitoneal involvement, and specific mesorectal excision or partial mesorectal excision (pME) for upper or middle third (intraperitoneal) tumors. In these cases, the distal margin should be 5 cm. The concept of 2 cm of margin in the non-irradiated rectum, or 1 cm of distal margin in irradiated recta, refers to the mucosal section margin for patients undergoing TME (Figure 7)24. TME can be performed by open, laparoscopic, or robotic surgery, depending on the surgeon’s expertise.
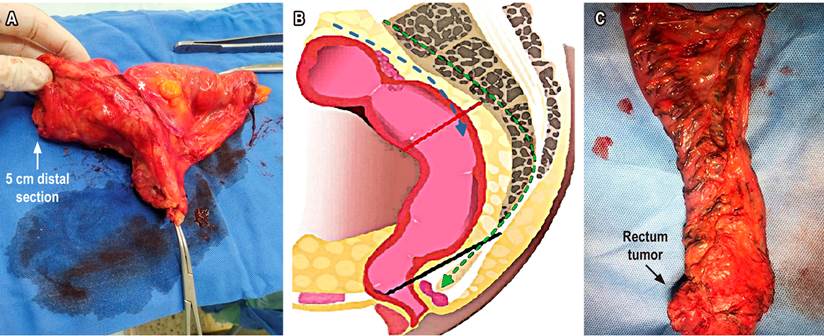
Figure 7 A. Specific partial or mesorectal excision (*tumor location). B. Mesorectal excision scheme. TME: green dotted lines to levator ani plane (black line). pME specific: blue dotted lines to distal section edge (red line). C. TME. Source: Authors’ file.
How is TME defined?
The rectum’s meso must be completely resected in the TME until the levator ani, through Waldeyer and Denonnvilier’s embryological fusion fascia. The surgeon and pathologist should evaluate the quality of this resection. The histopathologic report should describe whether the resection known as the mesocolic plane is complete, incomplete, or intramuscular. This characteristic has been defined as a prognostic factor of recurrence and OS.
In the last decade, a mesorectum approaching technique has been described in two ways: abdominal and perineal simultaneously. The direct transanal and transabdominal approaches are known as TATA. If the transanal approach is made with a laparoscopic perianal platform or device, the mesorectal dissection of the lower third is performed meeting the abdominal surgeon, the technique is known as transanal total mesorectal excision (TaTME)25. It is a developing surgical option for obese male patients, where performing TME up to the levator ani is not easy. Additionally, this technique ensures the distal section edge 26. It has defenders and detractors, and still, its oncological outcomes are under discussion 15,27.
Assessment of the presence of side adenopathies is a different concept in development and detectable only by MRI. These external nodes to the iliac vessels are currently considered a locoregional disease. They are not part of the conventional emptying of a TME. They are of special interest because, if they are detected by MRI and persist enlarged after radiotherapy, they have the indication of new surgery, known as pelvic lymph node dissection (PNLD). Lateral nodes are responsible for pelvic recurrence if this surgery is not carried out28.
What changed in the pathology?
The processing of the surgical specimen in macro is as important as the findings of the microscopic study. Surgeons tend to open the surgical specimen to check the safe section edges. However, this technically makes it difficult for the pathologist to evaluate the CRM. The ideal scenario is to preserve the piece in the tumor area and partially open it so that the formaldehyde enters and the edges can be processed correctly. This allows measuring the distance in millimeters from the T, the CRM, the distance to the MRF, the extramural venous invasion, and the quality of the mesorectum. All this, in addition to the nodes, their number, the degree of tumor differentiation, the tumor size, and the histological subtype. The pathology report should include, in detail, the previously described information (Figure 8)29.
Change in molecular biology
Knowing tumor biology helps determine prognosis and treatment. There are two ways to study mutations: on the piece and biopsies or blood, known as liquid biopsies. IMS instability is determined in the biopsy and the piece. The mutation in MSH, MLH, and PMS2 is measured as well as the status of the KRAS: mutated or native.
These important factors in the prognosis direct the oncologist on whether to use chemotherapeutics. IMS is associated with a poor response to fluoropyrimidines but with a better prognosis. Its presence supports the use of immunotherapy. The ras profile allows us to know whether biologics, such as epidermal growth factor (EGFR) inhibitors, should be used. In blood, the measurement of new carcinogenesis pathways (such as BRAF) will give treatment guidelines. Molecular staging is already a standard in studying rectal cancer patients 30.
Change in decision-making: multidisciplinary board
Ideally, decisions are made by an interdisciplinary team1, which should be composed of gastroenterology, colorectal or gastrointestinal surgery, radiology, oncology, radiotherapy, and pathology. The existence of this board will help define, from the beginning, the management and route for each patient (Figure 9).
Conclusions
Recent evidence makes the diagnosis, locoregional and systemic staging, and treatment of rectal cancer known by all specialties involved in its management. The different public and private institutions must make efforts, research, and clinical groups to implement the diagnostic and therapeutic approach strategies presented here. The exposed changes are in Figure 10.
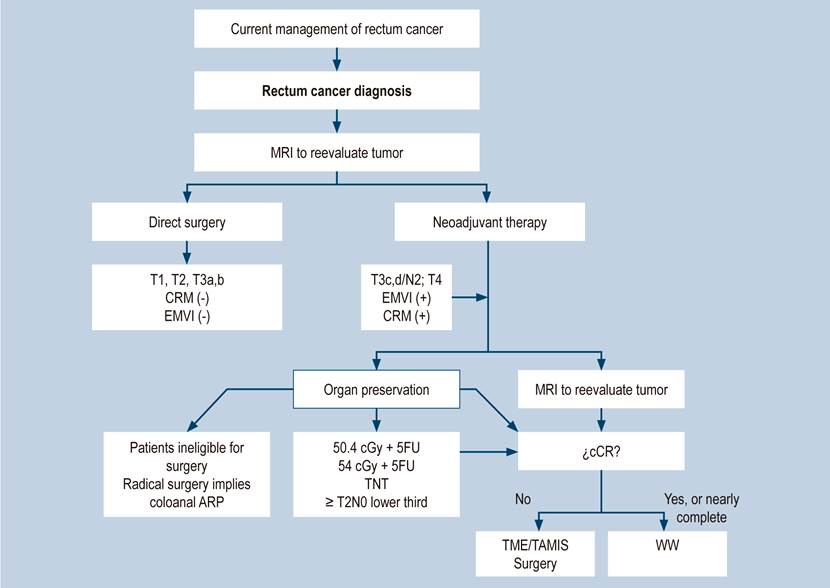
Figure 10 Flowchart for the current management of rectal cancer. 5FU: 5-fluoracil; cCR, complete clinical response; cGy: centigray; CRM: circumferential resection margin; EMVI, extramural vascular invasion; MRI, magnetic resonance imaging; TAMIS: transanal minimally invasive surgery; TME: total mesorectal excision; TNT: total neoadjuvant therapy; WW: Watch and wait. Source: Diagram made by authors.











 texto en
texto en