Introduction
Balantidium coli (B. coli) is the largest protozoan and the only ciliated parasite that infects humans1. Its distribution is global, but the greatest burden of infection is provided by developing countries2. In the Americas, most cases are reported in Central and South America3. The pig is the main animal reservoir, so the infection predominates in places where the man has close contact with pigs, a situation related to insufficient access to basic sanitation2,3. The disease caused by this protozoon is called balantidiasis2,4. We report the case of a schoolgirl who presented an intestinal perforation with perianal ulceration due to Balantidium coli, Enterobius vermicularis, and Trichuris trichiura coinfection.
Clinical case
A 7-year-old girl from a rural Indigenous community in Chocó, Colombia. She lives with her parents and 12 siblings in a dirt floor house with no electricity or drinking water services. The drinking water is directly obtained from the river. Unpasteurized dairy products are drunk, and pigs are raised. The patient was consulted for a 5-day-evolution clinical picture of subjective fever, diarrheal stools, and abdominal pain. After being evaluated in the local hospital, she was referred with a suspected diagnosis of dengue with warning signs. She was admitted in a septic shock status with an acute abdomen, so treatment with piperacillin-tazobactam was initiated. The initial blood count reported leukocytes 5000/ul, neutrophils 3900/ul, lymphocytes 800/ul, eosinophils 0/ul, hemoglobin 9.4 mg/dL, hematocrit 28.1%, platelets 60 000/ul, C-reactive protein (CRP) 22.8 mg/dL, and non-reactive HIV ELISA.
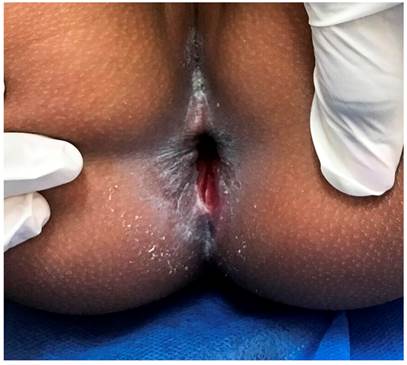
An exploratory laparotomy was performed, and fecal peritonitis caused by cecum perforation of the transverse colon (near the splenic flexure) was found, as well as 80% involvement of the ascending colon circumference by multiple microperforations. A right hemicolectomy was performed, with ileum resection at 5 cm from the ileocecal valve to the hepatic flexure, leaving the abdomen open. The patient was sent to the pediatric intensive care unit with vasopressor support. Intravenous metronidazole was added to the treatment. A perianal ulcer of 2 x 2 cm with a fibrin-covered bottom (Figure 1) was evidenced.
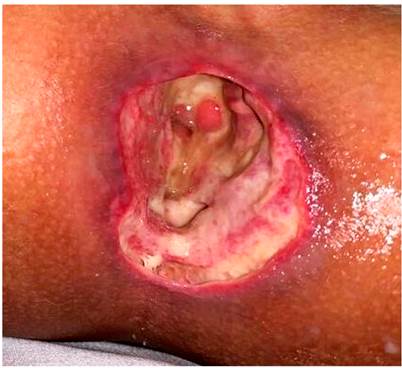
Figure 1 Perianal ulcer on admission to the institution. Approximate size of 2 x 2 cm. Note the disappearance of the anal sphincter’s anatomical relationships.
The patient was intervened after 2 days. Multiple perforations in the transverse colon and at a splenic flexure were found, with a collection in the right and subhepatic parietocolic gutter, ischemia points in the tapeworm, and antimesenteric border of rectosigmoid junction. A subtotal colectomy was performed with transverse colon, distal ileum, and sigmoid colon resection. The ileostomy was left. On day 5 of hospitalization, punctiform perforation raffia and peritonitis drainage were performed. On day 7, adhesions were released, and the intestine was repositioned in the abdominal cavity. A pathology report from colon samples taken in the first intervention was obtained. Parasitic infection by Balantidium coli (Figure 2)5 was confirmed, in addition to helminthiasis consistent with trichocephalus and pinworm. The patient had already received 7 days of metronidazole, so treatment for helminths with ivermectin (2 doses) was indicated by infectiology.
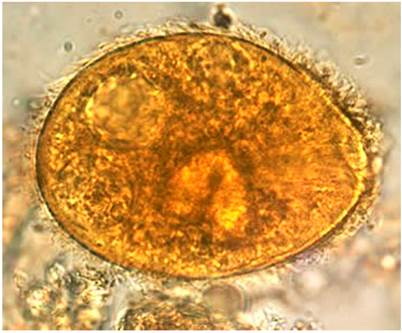
Figure 2 Balantidium coli: A trophozoite in a droplet of feces. Magnified x 1000. Note the visible cilia on the cell surface5. Image taken from5.
On day 10 of hospitalization, an exploratory laparotomy was carried out. Abdominal wall fasciitis, contained evisceration, ileostomy with fistula, and distal perforation of the wall were found. The abdomen was left open, and the perianal ulcer was debrided. After this intervention, there was clinical deterioration. An extension of the antibiotic spectrum to meropenem and vancomycin was required.
On day 15 of hospitalization, surgical lavage was carried out with vacuum-assisted closure (VAC) placement and mesh. A colon swab culture was obtained with yeast findings consistent with candida, so fluconazole was added to the treatment. The patient underwent surgery two more times. Final closure of the abdominal wall was achieved. The enteral route was then restarted, and oral metronidazole was received for 7 days. The perianal ulcer was healing with advanced cures using fitostimuline and hydrofiber dressings with silver. After 36 days of hospitalization, the patient was discharged with ileostomy, oral feeding tolerance, and multidisciplinary follow-up (Figure 3).
Discussion
Balantidium coli is the only ciliated protozoon known as a human pathogen. Its trophozoite is 50 to 200 μm in length and 40 to 70 μm in width. This microorganism causes balantidiasis, a zoonotic disease acquired by humans via fecal-oral transmission from its usual host: the pig. Contaminated water with porcine or human feces infecting cysts is its main vehicle, although it can also be acquired from person to person1,2.
The main risk factors for infection include close contact between pigs and humans, inadequate sanitation, and subtropical or tropical climates, which favor the parasite survival in the form of an infectious cyst2,3.
There are few reports in Latin America. A study in Bolivia examined more than 2000 stool samples in an asymptomatic population aged 5-19. B. coli was detected in 1.2% of cases6. Colombia has no prevalence records of this infection, but studies have been conducted on the Caribbean Coast. For example, Urbina et al identified Balantidium coli in 0.8% of children with acute diarrhea7.
The parasite has two forms: trophozoites, usually identified in dysenteric feces, and cysts in formed feces and chronic carriers. It is replicated by binary fission or sexual reproduction2. It inhabits the large intestine in humans and can remain there without invading tissues or causing clinical disease1,8. In fact, most infections are asymptomatic or occasionally cause acute diarrheal disease, a situation that accounts for 85% of patients who harbor the protozoan. This clinical form is more common in children than adults9. Less commonly, it can manifest with intermittent diarrhea, abdominal pain, and weight loss1.
In some cases, Balantidium coli can cause serious disease with dysentery, secondary to tissues destruction by invading the colonic mucosa. Along with hyaluronidase release, it causes ulcers and microabscesses associated with a polymorphonuclear inflammatory infiltrate1,2,10. This condition favors bacterial overgrowth, worsening the clinical picture11. Cases of extraintestinal dissemination have also been described in adults with genitourinary, pulmonary areas(12.13), and even vertebral osteomyelitis14, patients with immunosuppression(12.13), malnutrition(2.9), a history of excessive alcohol consumption15 or polyparasitism2.
Surgical intervention is required when invasive gastrointestinal complications such as typhlitis, appendicitis, and peritonitis occur9. Colon perforation is a sporadic complication associated with extremely high mortality rates1,3,16. Although the patient survived, she presented significant morbidity. She was taken to 8 surgical interventions with a complete colon resection, and a terminal ileostomy was necessary.
The treatment of choice for children over 8 years old is oral tetracycline at 40 mg/kg per day, divided into 4 doses for 10 days (maximum 2 g/day)17. As an alternative, since our patient was under 8 years of age, oral metronidazole was indicated in doses of 35-50 mg/kg/day, divided into 3 doses for at least 5 days17. However, it was not possible to start this treatment orally due to the patient’s condition. She received an initial intravenous cycle and then the oral one, which was successful. Another alternative is iodoquinol, 30 to 40 mg/kg per day (maximum 2 g) in 3 doses for 20 days18. Nitazoxanide may also be effective2. The literature reports other antibiotic agents that have been tested with variable success, such as paromomycin and chloroquine19.
In 2014, the “Clinical Practice Guide for the Promotion, Early Detection and Initial Approach of Growth and Developmental Disorders in Children”20 was published in Colombia. This guide recommends preventing intestinal parasitism in children with any epidemiological risk factor: food intake or contaminated water, life in rural areas, poor hygiene and education, human migrations, and poor socio-economic and health conditions. Some of these factors were identified in our patient. According to this guideline, these children should receive a single dose of albendazole, 400 mg orally. Although this drug is not recommended for the treatment of Balantidium coli, it is the first-line treatment of Enterobius vermicularis and Trichuris trichiura. It is likely that if albendazole had been administered with the semi-annual frequency established in the protocol, the girl would have had less risk of the presented outcomes. Thus, parasitic co-infection, one of the factors described in severe infections, would have been avoided.
This case provides evidence of the role of B. coli as a pathogen in immunocompetent children and of polyparasitism as a risk factor for severe complications, such as peritonitis with intestinal perforation, which are potentially preventable with deworming in at-risk populations. This is the first case reported in Colombia.
Conclusions
Infestation by intestinal parasites is common in children who do not have drinking water and live in poor health conditions, with chronic or recurrent diarrhea and abdominal pain as the predominant clinical manifestation. Perianal ulcers and intestinal perforation from parasitic causes are rare. Intestinal polyparasitism has a negative impact on the nutritional status of children. This, in turn, directly affects the tissue’s repair capacity so that intestinal ulcers can take longer to heal. The infestation by multiple parasites in the same patient can also favor a greater severity in symptoms since the pathogenicity mechanisms of each agent will be summative. It is important to guarantee the drinking water supply to at-risk populations, improve environmental sanitation conditions, diagnose and timely treat parasitic diseases, and correct the children population’s nutritional deficiencies, thus achieving their well-being and potential psychomotor, cognitive, and social development.











 text in
text in