Introduction
Non-Hodgkin lymphoma (NHL) of the gastrointestinal tract (GIT) is the most common extranodal lymphoma. Approximately 15%-30% of primary extranodal lymphomas occur in the GIT. Mantle cell lymphoma (MCL) accounts for 3%-6% of all NHL. It has a heterogeneous biological and clinical behavior and is frequently diagnosed at an advanced stage1,2.
Lymphomatous polyposis (LP) is a rare endoscopic finding that, although described typically in patients with primary and extranodal GI MCL, with GIT infiltration, is not specific to this type of lymphoma. It has also been described in patients with follicular lymphoma and mucosal-associated lymphoid tissue (MALT). LP is characterized by multiple polypoid lesions involving long segments of the GIT3,4.
We present the case of a patient with LP as an endoscopic manifestation of secondary involvement of the GIT by extranodal MCL. This case report was made following the case report guidelines (CARE)5.
Presentation of the case
A 62-year-old man with a history of hypertension (HT). He was admitted to the emergency room for a 4-month evolution clinical picture, consisting of non-painful adenopathies in the neck and inguinal region with progressive growth. During the last 2 months, he associates voice tone changes, mMRC 3 dyspnea, nocturnal diaphoresis with decubitus, oropharyngeal dysphagia for solids, and a 20 kg weight loss. On physical examination, the presence of up to 2 cm hard adenopathies is palpated in cervical, axillary, and inguinal lymph node chains. Bigger palatal tonsils are also felt. Initial paraclinical tests showed lymphopenia (900 μL) with no other abnormalities.
A contrasted tomography (CT) scan of the neck, abdomen, and pelvis suspecting lymphoproliferative disorder is performed. Tonsillar hypertrophy, which conditions the closure of 70% of the oropharynx, was found. Also, extensive involvement of adenopathies was found in cervical, axillary, and mediastinal lymph node chains, a subcarinal nodal conglomerate obstructing the main and retroperitoneal bronchi and gastric walls thickening (Figure 1).
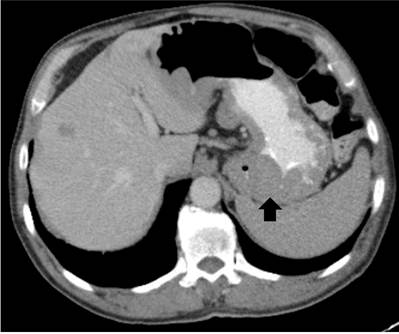
Figure 1 CT scan of the contrasted abdomen. Thickening of the gastric walls of predominance in the gastric fundus.
Upper gastrointestinal endoscopy (UGIE) with severely enlarged palatal tonsils, edematized esophageal mucosa, thickening of gastric folds, and presence of multiple polypoid, vascularized lesions, some superficially ulcerated between 2 and 25 mm (Figure 2).
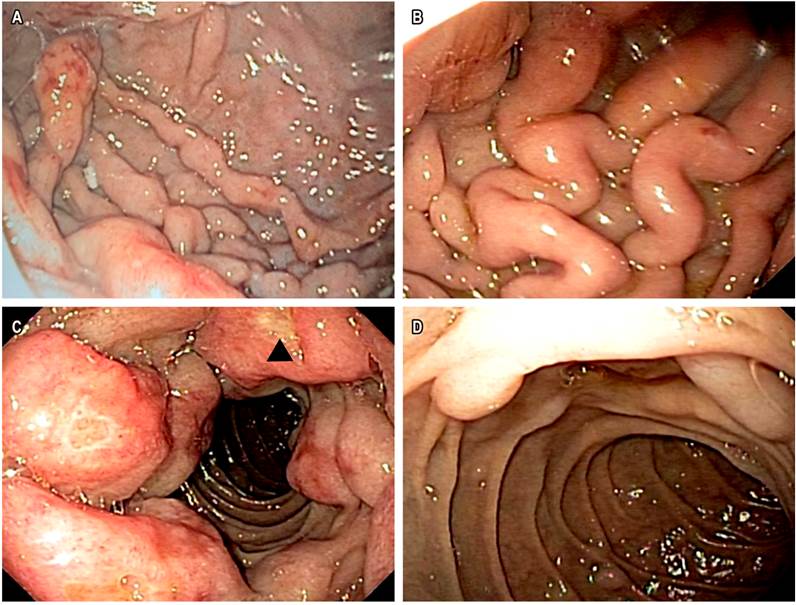
Figure 2 Upper gastrointestinal endoscopy A. Polypoid lesions on the gastric fundus. B. Severe thickening of gastric folds. C. Polypoid lesions with circumferential involvement of the duodenal bulb. Ulcerated lesion (∆). D. Polypoid lesions of variable sizes in second duodenal portion.
The esophagus, stomach, and duodenum histopathological study reveal an extensive infiltrate by atypical, mature, small-sized, and monotonic lymphocytes in the lamina propria. CD20, CD5, D1 cyclin, and bcl-2 positive with a 60% ki-67 in the immunohistochemical study with a compatible infiltration by classical MCL variant (Figure 3). Excisional biopsy of the cervical lymph node with similar findings. Bone marrow (BM) with 10% infiltration.
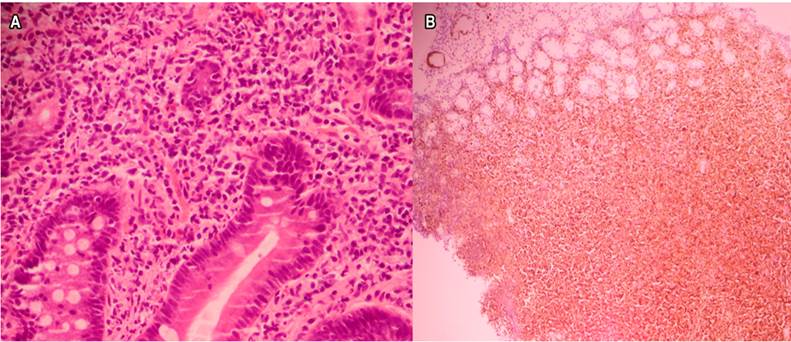
Figure 3 Histopathology and immunohistochemistry. A. Duodenal infiltration by small lymphocytes, monotonic (hematoxylin-eosin staining, 40x). B. Positive nuclear labeling for D1 cyclin (40x).
In a patient with nodal MCL, GIT and BM extranodal involvement, an intermediate risk mantle cell lymphoma international prognostic index (MIPI), and risk of complete airway obstruction by amygdalin infiltration, a systemic steroid was administered in the prephase. Then, chemotherapy was administered using the R-CHOP scheme (rituximab + cyclophosphamide + vincristine + doxorubicin + prednisone) interspersed with R-DHAP (rituximab + dexamethasone + cytarabine + platinum). The patient is currently in the fourth cycle of chemotherapy. Peripheral adenopathies have disappeared, and the tonsils’ size is now normal.
Literature review
MCL is a rare subtype of mature B-phenotype NHL. This represents 3%-6% of NHL. It predominantly affects men (2-3:1), and the mean age at diagnosis is 68 years1,2. At the time of diagnosis, 75% of patients present an advanced stage, with generalized lymphadenopathy, bone marrow, and GIT involvement1. GIT is the most affected extranodal site by peripheral MCL. There is microscopic involvement in 88%-92% of cases, but only 25% of patients present GI symptoms3,4,6,7. Endoscopic findings are variable and include polypoid lesions, granular patterns, superficial ulcers, diffuse infiltration of the GI wall, and nonspecific mucosal changes such as edema4,8.
LP was first recognized two centuries ago. However, it was described and reviewed for the first time in 1961 by Cornes et al9 as the primary GI involvement of the MCL. It accounts for 4% to 9% of primary GI B lymphomas and is originated from the mantle area of lymphoid follicles1-3. The main presenting symptoms are abdominal pain, diarrhea, and hematochezia. Weight loss, night sweats, and fatigue are also common10. The endoscopic finding consists of multiple polypoid lesions of 2 mm, up to several centimeters in diameter, with or without interspersed normal mucosa in one or more GIT segments. The proliferation of monomorphic small-to-medium size lymphocytes is characteristic of this polyposis, and in the study of immunohistochemistry like CD20+, CD5+, CD23-, with overexpression of chromosomal translocation t (11;14) and D1 cyclin3. It most often affects the ileocecal region, ileum, stomach, and duodenum10,11.
PL is the typical (uncommon) endoscopic pattern of GIT involvement by nodal MCL3,4,8,12. Esophageal involvement by MCL is unusual due to the low number of lymphoid cells3,13. Although GI involvement by MCL typically occurs as LP, this is not a specific finding of MCL. It has also been described in cases of T-cell NHL, follicular lymphoma, MALT, and diffuse large B-cell lymphoma3,4.
Primary GIT involvement by MCL in the form of LP has prognostic implications. It presents aggressive biological behavior and implies a median survival of 3 to 4 years3,4,6,12. In contrast, GIT evaluation, as disease staging in patients with confirmed nodal MCL, has little impact on therapeutic decisions1,3,4,6.
Treatment of LP involves the management of lymphoma. There is variability in clinical practice around the therapeutic approach to MCL. In general terms, the choice of treatment scheme is based on the patient’s characteristics (age, comorbidities, functional status), symptoms, staging, and safety profiles of the schemes. Chemoimmunotherapy, followed or not by consolidation with autologous hematopoietic cell transplant, is the usual approach1,2.











 texto en
texto en 



