Introduction
Food allergy occurs due to an immune-mediated response to one or more allergens. They can manifest clinically in different systems, including the cutaneous, respiratory, or gastrointestinal systems, reflecting individual susceptibility1. This allergy varies according to its immunological mechanism, leading to different phenotypes2. The more delayed non-IgE-mediated responses are more difficult to identify, unlike immunoglobulin E (IgE)-mediated food allergies. There are no simple tests to confirm the clinical suspicion. The golden test for diagnosis is the elimination of the allergen and the reintroduction or challenge, observing the improvement of symptoms upon removal of the allergen and their reappearance with reintroduction. Children under 2 years can go through this rigorous open food challenge (OFC). However, older people undergo the double-blind placebo-controlled food challenge (DBPCFC)3. This is an expensive process that requires training. Additionally, symptoms of the challenge can take several days to appear4.
The prevalence and incidence have increased over the years. The current series estimate a prevalence of 2% in the general population. In children under 5 years of age, the series varies from 1% to 10% and reflects IgE-mediated allergies5. However, for non-IgE-mediated allergies, the evidence is limited given the clinical variability, the delayed symptoms development after exposure, and the lack of standardized diagnostic criteria. Hence, these results in many studies have selection biases and a lack of sensitivity in case findings6.
Although each food could be considered a potential allergen, the list of those responsible, especially concerning the most severe reactions, is limited to a few food groups7. Cow’s milk allergy (CMA) is the most frequent. In a study conducted in Utrecht in 804 children, 7% had suspected (CMA) experiencing dermatological (71%), gastrointestinal (60%), respiratory (13%), and other (36%) problems. Fifty-six percent of the children underwent the oral food challenge test, but none had a proven allergy. Despite this, 71% were subjected to an elimination diet and the use of therapeutic formulas for long periods. In summary, it was necessary to improve diagnostic methods8.
These food allergies reportedly have a good prognosis. The resolution rate has been reported in 56% of children at 1 year, 77% at 2 years, 87% at 3 years, 92% at 5 years, and 97% at 15 years9. In a Finnish study, children with non-IgE-mediated reactions developed tolerance earlier than those with IgE-mediated responses, 64% versus 31% at 2 years and 96% versus 63% at 4 years10.
The economic burden of food allergy in the United States in 2007 was estimated at USD 510 million in total costs, with USD 340 million in direct costs per year. In addition, there were critical indirect costs with work absenteeism in parents and caregivers, which affected the quality of life11. No regional studies analyze the direct expenses of gastrointestinal food allergy.
In Colombia, the literature on gastrointestinal food allergy is scarce. Only one CMA study with a small population sample has been conducted12. The objectives of this study include describing demographic variables, clinical symptoms, nutritional status, established management, and natural history of the disease. Furthermore, to know the disease prevalence in the Colombian pediatric population (by age group) and to describe its direct costs.
Materials and methods
A study was conducted in 3 phases. The first phase consisted of estimating the prevalence of the disease through a query of the data from the Individual Registry of Health Services Provision (RIPS, by its abbreviation in Spanish) and My Prescription (MIPRES, by its abbreviation in Spanish). In the second phase, a retrospective descriptive study of the medical records of a cohort from a second-level pediatric gastroenterology reference center in Bogotá was conducted. Finally, in the third phase, a descriptive cross-sectional study was conducted on the selected cohort through telephone surveys. The protocol was approved by the ethics committee of Cayre Colombia, a healthcare provider.
The prevalence of the disease in Colombia was defined by consulting the database of the RIPS and MIPRES systems. The last 5 years were consulted age-by-age for those under 18 through the Integrated Information System of Social Protection (SISPRO, by its abbreviation in Spanish)13 using Excel® pivot tables. The ICD-10 K522 (allergic and dietary colitis and gastroenteritis) code was used. This code is the most closely related to the diagnosis of gastrointestinal allergy. The demographic information from the National Administrative Department of Statistics (DANE, by its abbreviation in Spanish) was used to estimate the national prevalence and prevalence by age group.
Between September 2011 and September 2020, the medical records were selected using the related ICD-10 codes, K-522 (allergic and dietetic colitis and gastroenteritis) and K20 (esophagitis). All patient records with a diagnosis confirmed by elimination-challenge test, or in the case of eosinophilic diseases, by histology results, were included. Ambiguous or incomplete medical records were excluded. The researchers reviewed all the records, filling in the data on a standard collection sheet to avoid errors. Relevant information was obtained on demographic data, clinical manifestations, time of evolution, birth type, first solid food, other personal or family allergies, nutritional status, diagnosis, management, and disease evolution. Direct costs were evaluated according to the number of pediatric gastroenterology consultations per year, endoscopies, colonoscopies, and allergy tests (specific IgE and skin prick test), based on healthcare providers (IPS, by its abbreviation in Spanish) contract prices. The median age of diagnosis and the median age of resolution were considered for the use of infant formula calculation. Eventually, the formula was adjusted for the average consumption of 2 cans per week, as previously published14. Parents or caregivers were interviewed through the telephone to find out the current status of the patients. They were asked about age and present symptoms, persistent need to avoid some food, presence of other allergic diseases, and parents’ opinion regarding the resolution of the disease.
The data were tabulated in an Excel® sheet. The RStudio software version 1.4.1106 was used for the descriptive analysis. All the variables were classified according to their category (numerical or categorical), and the respective distribution analyses were made for each.
Results
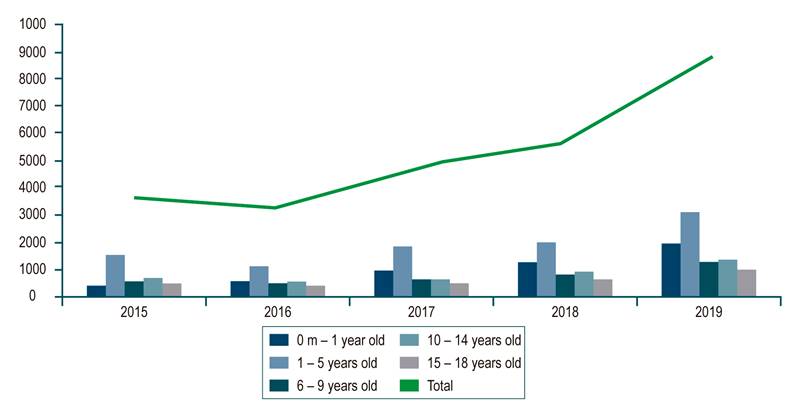
In the 2018 population census, the DANE published that Colombia had 48,258,494 inhabitants, including 23,501,887 people under 18. The RIPS database showed that 26,286 patients were reported from 2015 to 2019. The average prevalence of the investigated diagnosis in the population under 18 years of age was 0.02%. During the evaluated period, the group with the highest prevalence of allergic or dietary colitis and gastroenteritis was younger than 5 years, with an average of 0.074%. An analysis by year shows an increase in cases from 3,757 in 2015 to 8,807 in 2019 (all pediatric populations included [Figure 1]). The analysis in the MIPRES database of special formulas for children with the diagnosis studied reported an average prevalence of 0.006 % of formulated patients and a higher prevalence for children under 4 years of age, with 0.03 %. This is consistent with the RIPS report that shows an increase in formulation from 1537 products in 2017 to 4859 products in 2020 (Figure 2).
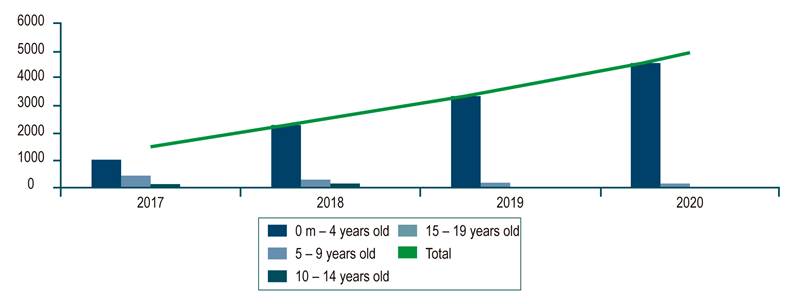
Figure 2 Special formulas for children diagnosed with allergic and dietetic gastroenteritis and colitis (number of products per year).
During the selected period, the IPS attended 7471 patients in the pediatric gastroenterology specialty. Medical records of 757 under the established ICD-10 codes were reviewed, and 472 (6.3%) were selected after applying inclusion and exclusion criteria, 219 (46.3%) were female, and 253 (53.6%) were male. The diagnoses were proctocolitis in 281 patients (59.3%), functional gastrointestinal disorders (FGIDs) in 63 (13.9%), eosinophilic esophagitis (EoE) in 51 (10.8%), immediate gastrointestinal hypersensitivity in 25 (5.3%), other eosinophilic diseases of the gastrointestinal tract in 9 (1.9%) and food protein-induced enterocolitis syndrome (FPIES) in 4 (0.84%). Figure 3 shows the population distribution by diagnosis, age, and gender. According to gender, the median age in allergic proctocolitis (APC) was 3 months (0.4-9), and in FGIDs, 3 and 4 months (1-15). Immediate gastrointestinal hypersensitivity and enteropathies showed a distribution between 12 and 24 months of life, and for eosinophilic diseases, the onset age was school age.
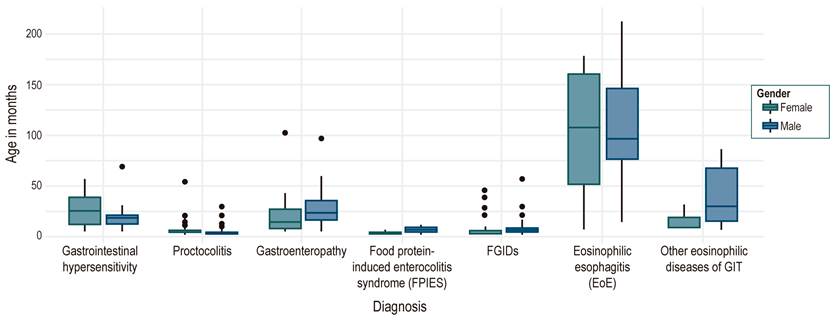
Figura 3 Distribution of the population by diagnosis according to age in months and discrimination by gender. FPIES: Food protein-induced enterocolitis syndrome, GIT: Gastrointestinal tract, FGIDs: Functional gastrointestinal disorders.
The main reasons for consultation were hematochezia in 275 patients (58.2%), diarrhea in 39 (8.26%), gastroesophageal reflux in 35 (7.41%), colic in 34 (7.2%), and others in 89 (18.8%). The median age at first consultation was 4 months, and most patients (75 %) were between 0.4 and 11 months old. Symptom evolution time at the first consultation showed a median of 3 months, most patients (85%) were seen before 6 months old. There were 272 patients (57.6 %) delivered by cesarean section, which was the dominant form of proctocolitis in 60 % of patients and FGIDs in 72 % of the patients (Table 1). Infant formula with CMP remained the first feeding at birth in 213 patients (61%) compared to 133 with breast milk (39%), distributed by diagnosis similar to birth type. Other allergic diseases reported on admission were atopic dermatitis (eczema) (13.3%) and asthma (3.8%). Most of the children (64%) had a family history of allergy. The type of feeding before the onset of the disease in infants was distributed as follows: Exclusive breastfeeding in 140 patients (29.6%), mixed breastfeeding in 225 patients (47.6%), and exclusive infant formula in 35 patients (7.4%).
Table 1 Diagnosis Related to Birth Type
| Vaginal Birth | Cesarean section | No information | |
|---|---|---|---|
| Immediate gastrointestinal hypersensitivity | 15 | 9 | 1 |
| Proctocolitis | 87 | 194 | 0 |
| Enteropathy | 22 | 13 | 1 |
| Food protein-induced enterocolitis syndrome (FPIES) | 3 | 1 | 0 |
| Secondary FGIDs | 18 | 47 | 1 |
| Eosinophilic esophagitis (EoE) | 7 | 4 | 40 |
| Other eosinophilic FGIDs diseases | 4 | 4 | 1 |
FPIES: Food protein-induced enterocolitis syndrome, GIT: Gastro-intestinal tract, FGIDs: Functional gastrointestinal disorders.
During the nutritional evaluation, the Z score for weight-for-age at the first visit showed a median of -0.64, height-for-age showed a median of -0.5 sd, and weight/height (W/H) in children under 2 years of age or body mass index (BMI) in older children had a median of -0.4 sd. A < -2 sd deficit was found specifically for weight/age in 13%, height/age in 10%, and W/H or BMI in 8%. At the first consultation, the W/H or BMI distribution by diagnosis and gender showed a greater nutritional involvement of enteropathies in females (Figure 4). In the follow-up, the last W/H or BMI Z score showed a median of -0.18, a ratio of patients with a (< -2 sd) deficit of 1.7%, and adequate nutritional management progress for the different types of diagnosis (Figure 5).
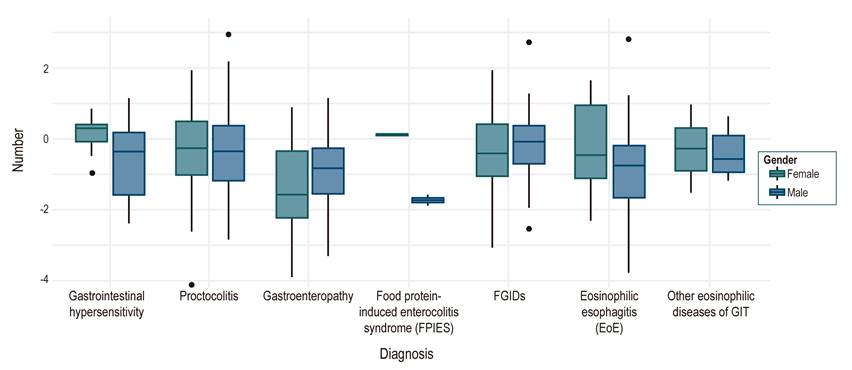
Figure 4 Distribution of the population according to the diagnosis by the weight/height ratio or BMI, discriminated by gender. FPIES: Food protein-induced enterocolitis syndrome, GIT: Gastrointestinal tract, FGIDs: Functional gastrointestinal disorders.
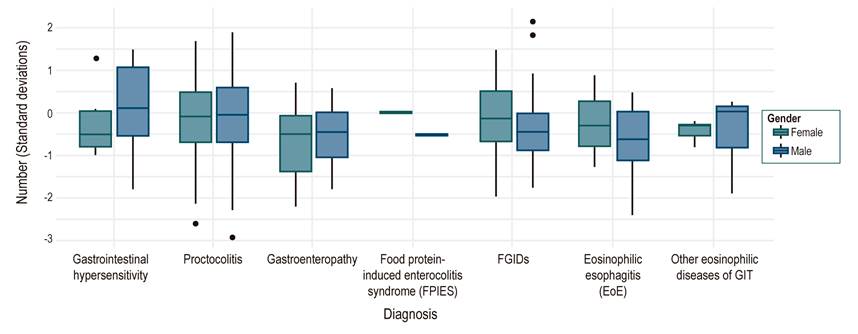
Figure 5 Distribution of the population according to the diagnosis by last weight/height ratio or BMI taken and discriminated by gender. FPIES: Food protein-induced enterocolitis syndrome, GIT: Gastrointestinal tract, FGIDs: Functional gastrointestinal disorders.
Dietary elimination of at least one allergen was required in 95.6 % of the population, where 91.3 % of the cases were CMA, followed by eggs in 4 %; in 14.6 % required 2 allergens elimination, and 5.5 %, 3 or more allergens were removed. Twenty-one patients who did not undergo dietary elimination belonged to the EoEs group.
Some 29.2 % of the patients had no therapeutic formula prescription; 334 patients received a therapeutic formula, including 149 with casein extensively hydrolyzed formulas (EHF), 88 with amino acid (L) formulas, 78 with serum EHF formulas, 11 patients with soy formulas, and 8 with hydrolyzed rice formula. Distribution by diagnosis in Table 2.
Table 2 Diagnosis and Use of Therapeutic Formulas
| EHF Casein | EHF Serum | EHF Rice | Soy F | AAF | No formula | |
|---|---|---|---|---|---|---|
| Immediate gastrointestinal hypersensitivity | 4 | 3 | 0 | 2 | 3 | 13 |
| Proctocolitis | 117 | 53 | 6 | 4 | 54 | 47 |
| Enteropathy | 4 | 6 | 0 | 5 | 6 | 15 |
| Food protein-induced enterocolitis syndrome (FPIES) | 1 | 0 | 0 | 0 | 2 | 1 |
| Secondary FGIDs | 22 | 16 | 2 | 0 | 14 | 12 |
| Eosinophilic esophagitis | 1 | 0 | 0 | 0 | 4 | 46 |
| Other GIT eosinophilic diseases | 0 | 0 | 0 | 0 | 5 | 4 |
Soy F: Soy-based formula, AAF: Amino acid formula; EHF Rice: Extensively hydrolyzed rice formula, EHF Cas: Extensively hydrolyzed casein formula, EHF Whey: Extensively hydrolyzed whey formula; FPIES: Food protein-induced enterocolitis syndrome, GIT: gastrointestinal tract; FGIDs: Functional gastrointestinal disorders.
The resolution age was established in 339 patients: Proctocolitis showed an 11-month median for both genders. FGIDs showed a median of 11 months for boys and 13 months for girls, and immediate gastrointestinal hypersensitivity showed a median of 24 months for boys and 43 months for girls. In enteropathies, there was a difference between genders with a later resolution age for males (a median of 45 months) compared to females (a median of 18 months) (Figure 6).
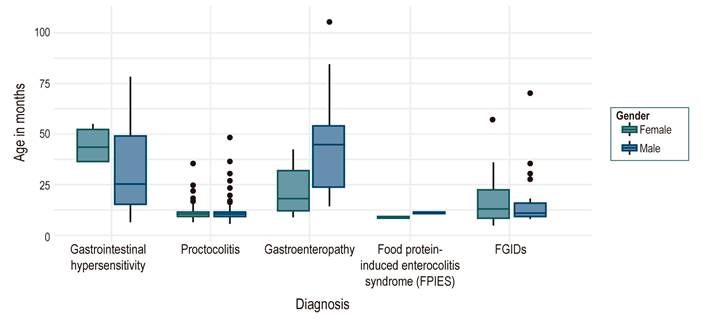
Figure 6 Distribution of the population according to diagnosis by resolution age and disaggregated by gender.
A total of 256 telephone surveys were conducted with a median age of 70 months (range: 10-293). The most frequent symptom was constipation (21.5%), followed by recurrent abdominal pain (9%), while 60% did not report any symptoms. Regarding the need to restrict some food in the diet, 87.5% said no dietary restriction, 7.4% reported CMP restriction, and 2.3% eggs, 20.7% of patients suffer from allergic rhinitis, 12% from atopic dermatitis, and 12.9% have 2 or more allergic diseases, 57.4% denied the presence of other allergies, 89% of parents reported resolution of their child’s food allergy.
The direct costs (Colombian pesos [COP], 2020) included the number of pediatric gastroenterology consultations per year, averaging 4, costing COP 160,000 per patient, 112 upper gastroenterology endoscopies for a unit cost of COP 321,000, equivalent to COP 76,170 per patient, 14 colonoscopies for a unit price of COP 425,000, corresponding to COP 12,606 per patient. Specific IgE measurement was performed in 121 patients with a cost per allergen of COP 74,000 for 6 allergens, equivalent to COP 113,822 per patient. Twenty-four patients took the skin prick tests for a unit cost of COP 180,000, equal to COP 9,152 per patient. Based on a 2-can consumption per week (8.5 cans per month), equivalent to 60 cans in total per patient, the average time of formula use was 7 months. Of 334 patients who received formula, 235 (70%) received HFE casein or serum, and 88 (26.3 %) received amino acid formula. Because these formulas were not being marketed at the time of the study and reference prices could not be obtained, 11 patients who received soy formula were not included in the cost analysis. The average price of the EHF for the period studied was COP 80,000 per can and COP 170,000 per can for the amino acid formula. In the cohort, the cost for the use of therapeutic formulas was COP 4,292,000 per patient. Total direct costs were COP 4,662,725 for each patient per year.
Discussion
Childhood food allergy is a chronic disease that significantly affects patients, parents, and caregivers’ quality of life. Although food allergies are one of the world’s most common chronic non-communicable diseases (NCDs), publications on disease burden and quality of life are scarce.
This study calculated the prevalence of gastrointestinal hypersensitivity according to the RIPS reports. Considering the report of the National Health Observatory, which conducted a comparative study between the RIPS and the 2010 National Demographic and Health Survey (ENDS, by its abbreviation in Spanish), is likely to be lower than the actual result, estimating a 50% underreporting by diagnosis in chronic non-communicable diseases15. The prevalence would be 0.04% for the pediatric population and 0.148% for children under 5 years of age if this expansion factor is applied. Similarly, there are no reports of population-based studies in the region to adjust this rate for underdiagnosis or misdiagnosis. A survey conducted in 2012 by the World Health Organization (WHO) reported that more than half of the countries surveyed did not have prevalence and incidence data, and only 10% performed an adequate diagnostic confirmation, while by parental report, a significant overestimation was observed.
Based on adequate diagnostic methods, a prevalence of 10% has been estimated in preschool children in developing countries and a marked increase in frequency in the last 10 to 15 years5. Similarly, we observed an increase of more than 100% in the number of cases reported between 2015 and 2019 in the RIPS. A lower prevalence of patients was observed compared to that reported in RIPS in the MIPRES database. Although, the formulation trend increased by 216% from 2017 to 2020. Based on the EuroPreVall cohort, which investigated school-age children in 8 European countries, parents reported adverse reactions to foods in 16.2% of children; however, confirmatory testing found only between 1.4% and 3.8%16. In a survey conducted in the United States to 38,408 parents and caregivers, the estimated prevalence of food allergy was 7.6%, with peanuts (2.2%), milk (1.9%), and shellfish (1.3%) as the most common allergens. Among allergic children, 43% presented at least one severe reaction and 39.9% showed a response to multiple allergens17. Evidence is even more limited for the prevalence of non-IgE-mediated food allergies given the clinical variability of presentation, the late symptoms development after exposure, and the lack of uniform diagnostic criteria6. Schoemaker et al. reported the incidence of allergy to CMP based on the EuroPreVall cohort at an adjusted rate of 0.74% during the first 2 years of life18. However, in this study, non-IgE-mediated allergy to CMP was not documented in 5 of the 9 participating countries that contributed 6,500 children to the study, which is why some authors considered a lack of sensitivity in the search process and selection bias19.
Our retrospective study focused on all gastrointestinal hypersensitivity phenotypes, and the diagnosis was confirmed in 6.3%. Vieira et al. reported a 5.4% allergy rate to CMP in children referred to gastroenterology centers in Brazil20. Meyer et al. reported 12.6% of non-IgE-mediated gastrointestinal hypersensitivities in a tertiary referral center21. This figure variability could relate to the type of reference center, phenotypes included, and potential regional differences. Half of our patients were in the first 4 months of age. An additional 25% were up to 11 months. This is consistent with the literature published in countries such as the United Kingdom and Brazil20,21. As previously published in a Colombian study of CMA, the most common digestive symptom was hematochezia (58.2%), followed by diarrhea 39 (8.26%), gastroesophageal reflux 35 (7.41%), and colic 34 (7.2%)12. The most common diagnoses were proctocolitis (59.3%) and secondary FGIDs, including gastroesophageal reflux disease and colic (13.9%), most of the frequent diagnoses in infants under 1 year of age. The majority of our population was in this age group. As previously reported, 50% of the patients were seen within the first 3 months of symptom onset in our clinic, with a short clinical course12,22,23.
However, the cohort report by Meyer et al. is striking. They evaluated patients in tertiary care with an average evolution of 63 months21. These differences may be related to the referral processes of health systems to more complex institutions. A delay in the time of diagnosis has been linked to a higher nutritional deficit, as reported in the Meyer study, in which 54% of the patients had poor growth. In our cohort, 13% of patients had a deficit greater than -2 sd in weight for age, a remarkably similar figure to that reported by Vera in Colombia and Vieira in Brazil with 15%12,20. Some 29.6% of the patients were under exclusive breastfeeding. Although reports of epidemiological studies of patients presenting CMA under exclusive breastfeeding are scarce, we still rely on Host et al.’s study, which revealed a prevalence of 0.5% of CMA in 1749 infants in 198824. Given the increase in frequency in recent years, a recent study investigating this field would be desirable.
As previously reported, cesarean delivery and the use of CMP formulas in the first 24 hours of life are more frequent, especially in patients with proctocolitis and FGIDs25.
The natural history of CMA has been previously evaluated. We found an 11-month resolution for patients with proctocolitis and FGIDs, which is consistent with the literature. We observed in our study a delayed resolution of enteropathy in males, even though it has a non-IgE-mediated mechanism, which could be affected by a more severe or extensive involvement18. Other factors affecting tolerance development include elevated IgE, multiple food allergies, and other allergic diseases such as asthma, rhinitis, and severe atopic dermatitis23. We found that 13% had atopic dermatitis and 3.8% had asthma, contrary to reports by Meyer et al., of 42% for atopic dermatitis and by Vera et al., who reported an association with other allergic diseases between 35% and 52% in the first year of life12,21. A likely reason for this difference is that in our series, we investigated the association of these other allergic diseases at the time of diagnosis, which was for most of our patients at 3 months of age. However, when investigating the disease evolution in the telephone survey, parents reported the presence of allergic rhinitis in 20%, and 13% had 2 or more allergic diseases. Sixty-four percent of our patients had a positive family history of allergy, a risk factor previously reported in the literature25. The primary allergen was CMA 91.3%, which was the most frequent allergy, as previously reported by Dierick et al26.
Of the patients who required formula use, 68% received EHF, and 26% received amino acids. The choice of the therapeutic formula is usually based on the physician’s decision. Although, among the factors that affect the formula selection include the patient’s age, risk of anaphylaxis, FPIES, severe allergy to CMP with complications such as malnutrition, anemia, or hypoalbuminemia, and adherence3.
Cow’s milk protein, egg, soy, wheat, fish, and nuts are among the most common allergens in pediatric EoE. Current dietary management focuses on eliminating 2 to 6 allergens, considering that very restrictive diets have a significant impact on nutrition, added to difficulties due to adherence27.
In monitoring current status by telephone interview, the most frequently reported symptom was constipation (21.5 %), followed by recurrent abdominal pain (9 %), similar to the prevalence reported in Colombia for the general pediatric population28. Functional constipation is estimated to account for 25% of consultations in a pediatric gastroenterology service. Less than 10% of patients require food allergens elimination in their diet, and 89% of parents reported the resolution of food allergy in their children, confirming the favorable evolution of food allergy with age, understanding that this varies according to its different phenotypes29.
A Turkish publication based on expert consensus estimated direct costs for patients with proctocolitis due to CMA over 2 years at USD 2116.05 and USD 2435.84 from the payer and societal perspectives, respectively. Direct costs included medical visits, laboratory tests, and treatment (formula or clinical nutrition). Eighty-nine percent of the cost is generated by food formula. In our study, the formula cost constitutes 92% of the direct costs evaluated. The second cost in our research was medical visits to the gastroenterologist (4 visits per year), which accounts for 3.4% of total costs. In the Turkish study, an average of 11 visits were made by patients, but other specialties were included; however, as in our research, it was the second highest cost30.
This study has several limitations. In calculating the prevalence from the RIPS and MIPRES registries, we could not establish an adjusted rate for underreporting or underdiagnosis. The information was collected from a second-level referral center for pediatric gastroenterology, which may not represent the Colombian population in general, particularly in the most severe cases of gastrointestinal hypersensitivity. A time horizon of 9 years in a field under continuous investigation can affect the results due to the changes generated in the approach of these patients. The study includes all the diagnostic phenotypes of gastrointestinal hypersensitivity, generating a high variability in the characterization data.
Conclusion
Thus far, this is the most extensive retrospective study published in Colombia, covering the demographic and clinical characteristics, management, and natural history of gastrointestinal food allergies. Hence, making information comparison with publications in other countries possible. Additionally, this study includes an analysis of prevalence and associated direct costs. Nevertheless, prospective and multicenter studies are needed in this ever-evolving field.











 texto en
texto en