Introduction
In recent years, the incidence of microscopic colitis (MC) has been increasing, as has the interest in studying this disease, about which little is still known. Potential incidence increases causes include the increasingly easy access patients have to colonoscopy procedures and the gastroenterologists and pathologists’ growing view and knowledge on the subject. In some populations, MC has reached and exceeded the incidence of inflammatory bowel disease (IBD), especially Crohn’s disease (CD)1,2.
This disease affects people over 60, especially females, with a female/male ratio of 9/1 in collagenous colitis (CC) and a lower proportion in lymphocytic colitis (LC)3. According to various studies, CC affects between 4.1 and 10/100,000 people/year. Lymphocytic colitis affects between 4.9 and 10/100,000 people/year; it is more common in whites and Jews and less common in Asians and Hispanics4.
As for its etiology, it is a multifactorial disease. Recently, it has been related to immunological factors; thus, some authors consider MC an initial IBD stage. A histological study confirms the diagnosis, but there is no consensus regarding the number of biopsies required. Some studies recommend 8 biopsies in each colonic segment to achieve the diagnosis5.
There are 3 types of microscopic colitis. First, in lymphocytic colitis, where chronic inflammation of the lamina propria is observed, the diagnostic criterion is the presence of more than 20 lymphocytes per 100 epithelial cells. The second is collagenous colitis, where the diagnostic criterion is the thickening of the collagen layer > 10 μm. Third, Incomplete microscopic colitis (MCi), including mixed symptoms typical of MC but no histological changes described. The ratio of lymphocytes to plasma cells is < 10/100, or the collagen layer is < 10 μm thick, which is why several researchers consider that the two forms initially mentioned do not correspond to two different diseases but to two different stages of the same disease5.
In Latin America, most publications related to this disease correspond to topic reviews or case presentations without determining the disease’s frequency6-9. The main objective of this study was to determine the number of microscopic colitis cases in a given period at Hospital Central de la Policía in Bogotá, Colombia, and, second, to evaluate the age and gender characteristics of patients diagnosed with MC in our health subsystem.
Materials and methods
Retrospective study of patient cases in which the pathology results of biopsies taken from all patients who underwent colonoscopy for a diagnosis of chronic diarrhea for 22 months, between February 2018 and November 2019, were reviewed and whose histological results confirmed the diagnosis of MC. We reviewed the gastroenterology service database, the pathology reports, and the medical records of the patients included in the study.
Inclusion criteria: patients over 18 years of age with a diagnosis of chronic diarrhea (more than 4 weeks) who underwent colonoscopy and biopsy, with the availability of the pathology report.
Exclusion criteria: patients who were not biopsied or whose pathology result was unavailable.
Variables analyzed: Gender, age, blood count findings, colonoscopy diagnosis, and histopathology.
This work was approved by the institutional committee of ethics and research and followed the current regulations of bioethical research.
Since this was a retrospective study, informed consent was not required from the patients based on the medical history review.
Results
In the 22 months between February 2018 and November 2019, 2,849 colonoscopies were performed in our institution for different diagnoses. Indications in 116 cases were chronic diarrhea. Chronic diarrhea was reported in 116 cases, in which biopsies were taken, and a histopathological report was available, resulting in 15 patients diagnosed with MC (Figure 1) due to the finding of lymphocyte infiltration in the lamina propria or due to thickening of the collagen layer. According to histological criteria, some microscopic colitis was found in 12.9% of patients who were biopsied for chronic diarrhea (Figures 2 and 3).
Figure 1 Histological findings of microscopic colitis in patients admitted for colonoscopy for chronic diarrhea.
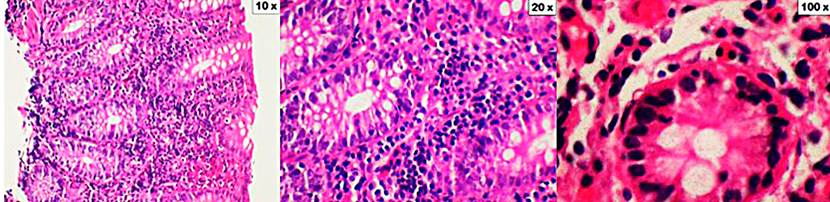
Figure 2 Lymphocytic colitis. The cuts show the mucosa of the colon distorted by a significant increase in intraepithelial lymphocytes (10x increase). In addition, there is a dense inflammatory infiltrate in the lamina propria consisting of lymphocytes, plasma cells, eosinophils, and occasional neutrophils (an increase of 20x and 100x). Coloration of hematoxylin and eosin (H&E).
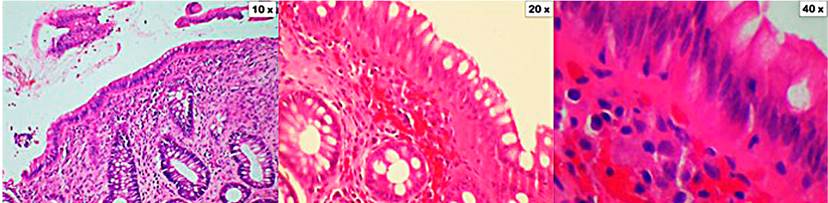
Figure 3 Collagenous colitis. The cuts show the mucosa of the colon distorted by the presence of a thickened subepithelial collagen band. Also, there are focal entrapment of capillaries, red blood cells, and inflammatory cells. In the lamina propria, there is a significant inflammatory infiltrate composed of lymphocytes, plasma cells, and eosinophils (10x, 20x, and 40x magnification). Coloration of hematoxylin and eosin (H&E).
Eighty percent of the patients (n = 12) were males, and only 3 patients were females. The median age was 47.5 years (range: 21-82 years) only 3 patients were older than 60. Colonoscopy was described as entirely usual in 60% of patients (n = 9), alterations were described in 6 of them, and only 2 patients received a clinical diagnosis of non-specific colitis. The diagnosis of MC was confirmed histologically in 15 patients: Three with MC, 1 with CC, and 11 with MCi (Table 1).
Table 1 Characteristics of Patients Diagnosed with Microscopic Colitis
| Patient | Gender | Age (years) | Diagnosis by Colonoscopy | Histopathology |
|---|---|---|---|---|
| 1 | Male | 37 | Normal | LC |
| 2 | Male | 31 | Non-Specific Proctitis | LC |
| 3 | Male | 35 | Normal | LC |
| 4 | Male | 64 | Normal | CC |
| 5 | Male | 82 | Normal | (Non-Specific) Colitis |
| 6 | Female | 53 | Normal | (Non-Specific) Colitis |
| 7 | Female | 48 | (Non-Specific) Colitis | (Non-Specific) Colitis |
| 8 | Male | 44 | Colorectal Polyp | (Non-Specific) Colitis |
| 9 | Male | 21 | Normal | (Non-Specific) Colitis |
| 10 | Male | 42 | Normal | (Non-Specific) Colitis |
| 11 | Male | 52 | Rectal Polyps | (Non-Specific) Colitis |
| 12 | Female | 58 | Normal | (Non-Specific) Colitis |
| 13 | Male | 35 | Normal | (Non-Specific) Colitis |
| 14 | Male | 35 | Rectal Polyps | (Non-Specific) Colitis |
| 15 | Male | 76 | Diverticula | (Non-Specific) Colitis |
Only one patient was found to have leukocytosis when reviewing the hemogram performed the month before the colonoscopy and biopsies. However, as in all the other patients, the total lymphocyte count was average (Table 2).
Tabla 2 Hemogram of patients with microscopic colitis
| Patient | Leukocytes | Hb | Platelets | Lymphocytes | % |
|---|---|---|---|---|---|
| 1 | 5478 | 15.49 | 214 200 | 1890 | 33 |
| 2 | 7550 | 15.2 | 235 000 | 2570 | 34 |
| 3 | 6430 | 15.8 | 364 000 | 2780 | 43 |
| 4 | 11 750 | 15.06 | 525 700 | 2560 | 21 |
| 5 | 6510 | 16.9 | 216 000 | 1820 | 27 |
| 6 | 9060 | 18.1 | 219 000 | 3200 | 35 |
| 7 | 8280 | 15.67 | 237 800 | 1910 | 23 |
| 8 | 7100 | 16.4 | 261 000 | 2190 | 30 |
| 9 | 6250 | 15.1 | 281 000 | 2240 | 35 |
| 10 | 7980 | 17.1 | 261 000 | 2650 | 33 |
| 11 | 8540 | 17.3 | 167 000 | 3260 | 38 |
| 12 | 7990 | 13.3 | 194 000 | 2600 | 32 |
| 13 | 6830 | 16.9 | 153 000 | 1770 | 25 |
| 14 | 3757 | 16.3 | 140 000 | 1320 | 35 |
| 15 | 8120 | 16.3 | 203 000 | 2040 | 25 |
Hb: Hemoglobin
Discussion
On the one hand, chronic watery diarrhea can occur due to various organic diseases, including IBD, MC, infections or intestinal bacterial overgrowth, and colon cancer. On the other hand, functional alterations can also cause this type of diarrhea, including Functional Diarrhea (FD) and Diarrhea-Predominant Irritable Bowel Syndrome (IBS-D). Current criteria for the diagnosis of these two entities are those described in the Rome IV classification and differ from each other by abdominal pain that is generally absent in FD and is very intense in IBS-D. Because many of the symptoms of these 2 diseases are similar to those caused by organic-type diarrhea, the final diagnosis is made by exclusion3.
Currently, many authors include MC within the IBD group, alongside such significant pathologies as Chron’s disease (CD) or ulcerative colitis (UC). However, it presents very different ages, symptoms, disease evolution, and treatment characteristics. Crohn’s disease and UC are often considered systemic diseases, affecting not only the colorectal region but also frequently associated with other diseases and neoplastic complications, both of which have not been demonstrated concerning MC10.
This disease was first described in 1976 by Lindström, who reported the case of a patient with watery diarrhea and CC. In 1982, Lazenby introduced the terms LC and MC. Epidemiological studies have shown an increasing incidence and prevalence, reaching levels similar to those of CD and UC. The incidence of MC has been increasing in the United Kingdom and the United States, although it appears to have stabilized in the latter11.
Microscopic Colitis is characterized by non-bloody watery diarrhea associated with cramping-like abdominal pain and occasionally weight loss. It occurs mainly in adults over 60 and is more common in women. An incidence rate of approximately 10/100,000 people per year has been reported11.
In this study, 2 results were found opposite to what was mentioned in the literature: Eighty percent of the cases (n = 12) corresponded to male patients. Most patients (n = 12) were under 60 years old.
Pathophysiology
The causes of MC have not been clearly defined, and it is considered a multifactorial disease. Immune response, genetic susceptibility, and changes in epithelial barrier function have been implicated. These alterations promote increased mucosal permeability to antigens and bacteria. They facilitate inflammatory processes in the lamina propria, associated with the severity of symptoms, especially diarrhea, due to a decrease in sodium chloride absorption and active chloride secretion4.
Some studies have linked MC to bile acid malabsorption (BAM). A Spanish study found that BAM was present in 43% of patients with MC, more commonly in LC (60%) than in CC (27%). Although cholestyramine treatment was met in 86% of patients, other studies have not demonstrated the same effect12 Furthermore, MC has been linked to the human leukocyte antigen (HLA)-DQ2, which is associated with other autoimmune diseases and alterations of the fecal microbiota (depletion of the bacterium Akkermansia muciniphila)10.
Risk Factors
In a systematic review and meta-analysis evaluating cigarette smoking as a risk factor, most studies found that smoking is a predisposing factor. Overall, patients who smoke are 3 times more likely to develop MC than non-smokers13.
There are hypotheses involving the change of the gut microbiota in smoking patients, which leads to a dysbiosis that alters the epithelial barrier in the mucosa, contributing to the onset of diarrhea. Other factors such as alcohol intake and dietary factors have been mentioned13.
Autoimmune disorders such as rheumatoid arthritis, thyroiditis, and celiac disease have been linked to the onset of MC. Furthermore, female hormonal factors and the consumption of medications such as nonsteroidal anti-inflammatory drugs (NSAIDs), proton pump inhibitors (PPIs), selective serotonin reuptake inhibitors, and low doses of aspirin have also been linked to MC onset14.
Patient-related risk factors were not established because this is not the objective of this study and because of incomplete records of medical histories.
Diagnosis
Many clinical guidelines focus on the treatment of diarrhea. Although, the guidance regarding the diagnostic approach is generally poor. Therefore, it can lead to the improper use of diagnostic tests, causing loss of time, unnecessary expenses, discomfort in the patient, and inaccurate results. Unfortunately, MC remains largely unknown and is overlooked by front-line physicians, including some specialists, when treating patients with chronic diarrhea.
Microscopic Colitis displays a spectrum of symptoms ranging from mild, self-limiting episodes of diarrhea to debilitating and severe abdominal pain episodes, joint pain, fatigue, and weight loss. However, in no case has it been shown to have a mortality risk, nor has it been associated with the development of colorectal cancer. It significantly affects patients’ quality of life, with an impact comparable to that of ulcerative colitis15.
Through medical history, diseases with similar symptoms such as IBD, celiac disease, and IBS-D can be ruled out. Laboratory tests, including hemograms, are usually standard, as in the case of all our patients, and similar to radiological studies, can help rule out other diseases. Non-specific changes such as elevation of C-reactive protein (CRP) and anemia can be found. Fecal calprotectin and lactoferrin have low diagnostic accuracy. Sometimes subtle mucosal changes such as edema and altered vascular pattern may be observed, but colonoscopy is usually regular, which is the case in most of our patients16. Our setting recommends colonoscopy with biopsies for all chronic diarrhea patients.
A systematic review and meta-analysis evaluating 10 studies with more than 3900 cases found a combined prevalence of IBS-D symptoms in 33.4% of people with MC. Diagnosis should be based on the patient’s clinical characteristics and confirmed by histology of step biopsies of the colon, minimum of 2 in each segment17. The number of segments and biopsies taken in our institution depends on each gastroenterologist’s criteria and the procedure’s findings. Samples of 3 segments (right, transverse, and left) are usually routinely taken.
Chronic inflammation of the lamina propria is observed in LC due to a proliferation of plasma cells, a decrease in the number of goblet cells, and the infiltration of more than 20 lymphocytes per 100 epithelial cells. In CC, the collagen layer thickens, which exceeds the standard upper limit of 7 μm. However, some authors consider thickening > 10 μm as diagnostic. In addition to these 2 histological subtypes, there is MCi, which corresponds to the presence of clinical symptoms suggestive of the disease, with histological findings that do not meet the criteria above. In these cases, the number of lymphocytes and plasma cells is < 10/100 epithelial cells, and the subepithelial collagen is < 10 μm. These findings suggest that the 2 classic forms of MC may not correspond to 2 different diseases but 2 different stages in developing the same disease5,10. The above described corresponds to the diagnostic criteria used in the pathology department of our hospital.
So far, no specific biomarker has been found to assess disease activity. Indicators have been proposed that include the number of loose or liquid stools per day, nocturnal stools, abdominal pain, weight loss, and fecal urgency or incontinence. There is a direct correlation with MC and an indirect correlation with IBD. A study of 116 patients with CC found that patients had 3 or more stools per day, or one or more watery-looking stools, representing a negative effect on the patients’ life quality18.
Treatment
One of the fundamental pillars is eliminating all MC risk factors such as smoking, caffeine, dairy, and alcohol intake. Also, medications are recommended, especially aspirin, NSAIDs, lansoprazole, omeprazole, ranitidine, sertraline, and ticlopidine. Celiac disease and bile acid malabsorption (BAM), which may coexist with MC, should be ruled out19.
Budesonide has been recommended as the medicine of choice in the treatment of MC for more than a decade. Medications such as prednisolone, mesalazine, and bismuth subsalicylate are second-line agents. Several randomized studies agree on the efficacy of budesonide in inducing remission in CC and CL. Meta-analyses have confirmed its effectiveness in controlling active MC, showing improvement before 2 weeks, given the absence of diarrhea. Therefore, the treatment of MC 20) has been recommended with this drug, the one our patients take. Responsiveness and monitoring were not analyzed as this was not the study’s objective.
Twenty-eight patients were randomized in a double-blind placebo-controlled food challenge (DBPCFC) study evaluating the effectiveness of budesonide in patients with CC. Half of them received a placebo, and half received 3 capsules of 3 mg Budenofalk® (9 mg/day) for 8 weeks. A satisfactory response (50% decrease in stool quantity at week 8) was observed in 8 of 14 patients treated with budesonide compared to 3 of 14 placebo responders (p = 0.05). Histological follow-up was performed by comparing biopsies at weeks 0 and 8, with no changes in mean collagen band thickness but a significant decrease in lamina propria infiltration (p < 0.001). It was concluded that budesonide effectively induces a short-term clinical response in CC21.
To compare the efficacy of budesonide and mesalazine in CC, a multicenter phase 3 study was conducted in 31 European centers, in which 92 patients were randomized into 3 groups (Budenofalk®, Salofalk®, or placebo) and a histological and clinical improvement (better stool consistency and improvement of abdominal pain) was observed in 80% of patients receiving budesonide, compared to 44% of patients receiving mesalazine (p = 0.0035)22.
Currently, budesonide is recommended by the American Gastroenterological Association (AGA) as first-line therapy. Other studies have also demonstrated effectiveness in LC and maintenance of clinical remission with budesonide at doses of 4.5 mg for 12 months23-26.
Azathioprine, 6-mercaptopurine (6-MP), and some biologics (anti-tumor necrosis factor alpha [anti-TNF-α]) have been used in microscopic colitis, especially in patients with refractory symptoms or steroid dependence. Loperamide at 2-16 mg/day helps control symptoms19.
Corticosteroids are only recommended in patients refractory to budesonide treatment when budesonide is unavailable, and other etiologies such as celiac disease have been ruled out26.
There is no consensus as to the criteria for referral. Each treatment must be individualized, determining to what point to extend it. Short-term treatment should be 6 to 8 weeks and continue for up to 12 months to avoid relapses27.
Surgical treatment should be the last alternative and reserved only for patients who do not respond to medications. Small series have been reported in which subtotal colectomy, Ileoanal anastomosis, or ileal pouch-anal anastomosis (IPAA) have been performed28.
As a possible bias, we should consider that it was impossible to view the total number of patients with chronic diarrhea assessed in the various consultations performed on patients at our institution. Furthermore, given that this was a retrospective study, it was impossible to evaluate the presence of associated risk factors explaining the high figures found.
Conclusions
In contrast to what is reported in the literature, we found that microscopic colitis affected especially young patients with a mean age of 47.5 years in this study. In addition, an unusual finding was that the male/female ratio was 4/1.
According to histological criteria, some microscopic colitis was found in 12.9% of patients biopsied for chronic diarrhea, a very high figure considering the incidence usually reported.
The objective of this study was to identify the presence of the disease in our population group. Given the findings, it is recommended to conduct prospective studies considering patients’ history, treatment, and follow-up.











 texto en
texto en 



