Introduction
Infection by the severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) called COVID-19 pandemic infection (from the English novel coronavirus disease 2019) is receiving the most attention now, and liver involvement is not alien to the viral infection. Asymptomatic elevation of transaminases is typical, and liver injury varies from 14% to 78%1-5. Gastrointestinal manifestations such as nausea, vomiting, abdominal pain, diarrhea, loss of appetite, dysgeusia, and liver chemical changes are increasingly being reported, especially in hospitalized patients4,5.
In the meta-analysis by Dorrell et al5, 62 studies were found that reported transaminase alterations with average alanine aminotransferase (ALT) of 34.8 (+/-16.1 U/L) and aspartate aminotransferase (AST) 39.0 U/L (+/-17.3) among all patients with coronavirus disease 2019 (COVID-19), with a weighted average AST:ALT ratio of 1.15 (+/-0.20).
Liver damage may be directly caused by virus-induced cytopathic effects, considering that SARS-CoV-2 binds to the angiotensin-converting enzyme 2 (ACE-2) receptor to enter its target cells6,7. Data from two independent cohorts revealed a significant enrichment of ACE-2 expression in cholangiocytes (59.7% of cells) compared to hepatocytes (2.6% of cells), suggesting that SARS-CoV-2 could bind directly to ACE-2-positive cholangiocytes and produce alterations in liver function8-10.
Among the liver diseases described and associated with severe cases of COVID-19, the main one was chronic hepatitis B infection, with 2.4% of severe cases. AST elevation is observed in approximately 18.2% of non-severe cases and 39.4% of severe cases, and ALT elevation in 19.8% of non-severe cases and 28.1% of severe cases.
Few studies have depicted hepatic manifestations, with one study in Wuhan, China, where the origin of the virus was initially described in a series of 99 patients. A decrease in albumin was observed in 98% of patients. At the same time, serum levels of AST, ALT, and bilirubin were elevated in 35%, 28%, and 18% of patients, respectively11. Similarly, in an analysis of 1,099 patients, elevated AST levels were noted in 18.2% of patients with non-severe disease and 39.4% of patients with severe disease12.
Therefore, considering that the description of the hepatic manifestations of COVID-19 is scarce and that there is no record thereof in Colombia, the present study aims to explain the behavior of a cohort of patients with liver diseases who suffered from COVID-19.
Materials and methods
A retrospective observational study was conducted with a review of medical records that analyzed the behavior of a cohort of patients with liver disease who became ill with COVID-19.
Only patients older than 18 years who were under follow-up for liver disease at the Center for Liver and Digestive Diseases (CEHYD) from March 2020 to June 2021 were included. Pregnant patients and those under 18 years of age were excluded.
Statistical analysis
The variables of interest were compared with a description of the sociodemographic variables through frequency and central tendency measures. The variables with normal distribution were compared using the chi-square test, while the variables with abnormal distribution were analyzed using the Mann-Whitney test. Survival analysis was performed by comparing the variables related to mortality represented in Kaplan-Meier graphs and using the log-rank test. Results were presented as tables or graphs using Stata 13.0.
Results
We reviewed a total of 1,937 medical records of patients under follow-up for liver disease with suspected SARS-CoV-2 infection, confirming the diagnosis by molecular tests (real-time polymerase chain reaction [RT-PCR] or antigens) in 543 patients, of whom 300 were women (55.3%).
The median age at diagnosis of liver disease was 52 years (interquartile range [IQR]: 40-61). The main associated comorbidities were high blood pressure (HBP) (23%), dyslipidemia (20.1%), and obesity (17.6%), and the leading causes of liver disease were, in order, non-alcoholic steatohepatitis (NASH; 49.5%), cholestatic disease (7.7%), hepatitis C and B virus (6.3%), and alcohol (4%), with a significant difference between them. Sociodemographic characteristics are shown in Table 1.
Table 1 Sociodemographic characteristics and laboratory variables in all patients
| Variable | Total n = 543 n (%) | Women n = 300 (55.3 %) n (%) | Men n = 243 (44.7 %) n (%) | P-value |
|---|---|---|---|---|
| Sociodemographic characteristics | ||||
| - Age at diagnosis Median (IQR) | 52 (40-61) | 53 (40-61) | 51 (39-62) | 0.628* |
| Background | ||||
| - DM | 67 (13.3) | 39 (13.8) | 28 (12.6) | 0.7ç |
| - Dyslipidemia | 103 (20.1) | 52 (18.1) | 51 (22.6) | 0.2ç |
| - Obesity | 90 (17.6) | 45 (15.6) | 45 (20.0) | 0.2ç |
| - Alcohol use | 204 (37.5) | 60 (20) | 144 (59.2) | <0.001+ |
| - HBP | 118 (23.0) | 66 (23.0) | 52 (23.1) | 0.976ç |
| - Coronary heart disease | 26 (5.0) | 15 (5.2) | 11 (4.8) | 0.863ç |
| - BMI Me (IQR) | 26.5 (24.29.4) | 26 (23-29) | 27 (24.9-30) | 0.002* |
| Laboratories (n = 151) | Median (IQR) | |||
| - Leukocytes Me (IQR) | 5950 (5000-7230) | 5905 (4762-7157) | 6190 (5070-7295) | 0.2352* |
| - Neutrophils | 54 (47-60) | 54 (48-61) | 52 (46-59) | 0.0621* |
| - Lymphocytes | 34 (27-39) | 33 (28-39) | 34 (26-41) | 0.2057* |
| - ESR (mL/h) | 7 (3-15) | 8 (5-20) | 5 (2-8) | <0.0001* |
| - Hb | 15 (14-16) | 14 (13-15) | 16 (15-17) | <0.0001* |
| - HCT | 45 (42-48) | 43 (41-46) | 48 (45-50) | <0.0001* |
| - Platelets | 242000 (192 x103-288 X103) | 260000 (207 x103-307x103) | 218000 (178 x103-262 x 103) | <0.0001* |
| - Glycemia | 94 (86-102) | 91 (84-100) | 96 (89-104) | <0.0001* |
| - Creatinine | 0.8 (0.7-1) | 0.7 (0.6-0.8) | 0.9 (0.8-1.1) | <0.0001* |
| - Total cholesterol | 193 (160-224) | 196 (166-229) | 188 (153-222) | 0.0319* |
| - Triglycerides | 143 (104-194) | 138 (93-184) | 147 (113-204) | 0.0106* |
| - TSH | 2.5 (1.5-3.6) | 2.5 (1.5- 3.6) | 2.4 (1.6-3.7) | 0.7767* |
| Liver function | ||||
| - AST | 35 (23-62) | 33 (21-63) | 35.5 (26-61) | 0.1763* |
| - ALT | 52 (30-98) | 49 (23-101) | 58 (36-95) | 0.0064* |
| - Alkaline phosphatase | 96 (74-136) | 101 (77-142) | 91 (72-134) | 0.0279* |
| - Total bilirubin | 0.7 (0.4-1.1) | 0.6 (0.4-1) | 0.7 (0.5-1.3) | <0.0001* |
| - Albumin | 4.4 (4.1-4.7) | 4.4 (4.1-4.6) | 4.5 (4.2-4.8) | 0.0007* |
| - AFP | 2.5 (1.7-3.9) | 2.3 (1.6-3.6) | 2.7 (1.7-4.3) | 0.0882* |
| Cause of liver disease | <0.001+ | |||
| - Fatty liver | 269 (49.5) | 135 (45) | 134 (55.1) | |
| - Alcohol | 22 (4.1) | 3 (1) | 19 (7.8) | |
| - Virus | 34 (6.3) | 19 (6.3) | 15 (6.1) | |
| - Cholestatic | 42 (7.7) | 36 (12) | 6 (4.5) | |
| - Mixed (three or more) | 14 (2.6) | 7 (2.3) | 7 (2.8) | |
| - Other | 162 (29.8) | 100 (33.3) | 62 (25.5) | |
çChi-square. *Mann-Whitney test. +Fisher’s test. AFP: Alpha-fetoprotein; DM: Diabetes mellitus; Hb: Hemoglobin; HCT: Hematocrit; Me: Median; TSH: Thyrotropin; ESR: Erythrocyte sedimentation rate.
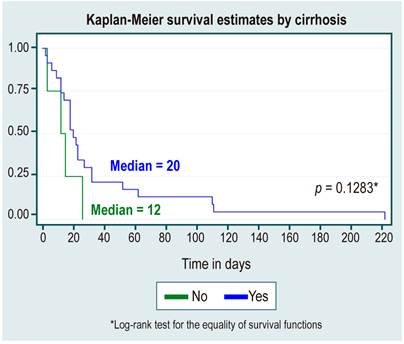
One hundred fifty-two patients (27.9%) were diagnosed with cirrhosis with statistically significant differences (p < 0.001), together with coronary heart disease (p < 0.015) for mortality.
The leading cause of cirrhosis was again NASH in 36.8% (n = 42), and 28.2% had a history of decompensation, and the most frequent were ascites (40%), followed by variceal bleeding (20 %).
Transaminases had higher values for ALT, showing a median of 52 U/L (IQR: 30-98), and for AST, there was a slightly elevated value with a median of 32 U/L (IQR: 23-62). Laboratory variables were taken before a diagnosis of SARS-CoV-2 infection.
Despite not having statistically significant differences in mortality, a higher proportion of DM, dyslipidemia, and obesity stands out in patients who survived, as shown in Table 2.
Table 2 Variables related to mortality from COVID-19 in patients with liver disease
| Variable | Total n = 543 n (%) | Alive n = 512 (94.3) n (%) | Deceased n = 31 (5.7) n (%) | P-value |
|---|---|---|---|---|
| Clinical features | ||||
| - Women | 300 (55.3) | 283 (55.2) | 229 (44.7) | 0.962ç |
| - Diabetes (n = 503) | 67 (13.3) | 63 (13.3) | 4 (12.9) | 1+ |
| - Dyslipidemia (n = 512) | 103 (20.1) | 99 (20.5) | 4 (12.9) | |
| - Obesity (n = 511) | 90 (17.6) | 87 (18.1) | 3 (9.6) | |
| Alcohol use | 204 (37.5) | 188(36.7) | 16 (51.6) | 0.001 |
| - Does not use | 302 (59.6) | 287 (60.4) | 15 (48.3) | |
| - HBP | 118 (23.0) | 111 (23.0) | 7 (22.5) | 0.949ç |
| - Coronary heart disease | 26 (5.0) | 21 (4.3) | 5 (16.1) | 0.015+ |
| - Cirrhosis | 152 (100) | 74 (100) | 78 (100) | <0.001ç |
| Cause of cirrhosis | 0.274+ | |||
| - NASH | 42 (36.8) | 29 (39.1) | 20 (25.6) | |
| - NASH + alcohol | 10 (8.7) | 1 (1.3) | 16 (20.5) | |
| - Hepatitis C virus | 8 (7.0) | 16 (21.6) | 5 (6.4) | |
| - Autoimmune | 8 (7.0) | 6 (8.1) | 1 (1.2) | |
| - Alcohol | 7 (6.1) | 1 (1.3) | 23 (29.4) | |
| - Other causes | 20 (15.6) | 8 (10.8) | 12 (8.9) | |
| Decompensation | 0.005+ | |||
| - Ascites | 12 (20.3) | 6 (13.6) | 6 (40.0) | |
| - Variceal bleeding | 8 (13.5) | 5 (11.3) | 3 (20.0) | |
| - Encephalopathy | 3 (5.0) | 1 (2.2) | 2 (13.3) | |
| - HCC | 2 (3.3) | 1 (2.2) | 1 (6.6) | |
| - Jaundice | 7 (11.8) | 6 (13.6) | 1 (6.6) | |
| - Coagulopathy | 3 (5.0) | 2 (4.5) | 1 (6.6) | |
çChi square. *Mann-Whitney test. +Fisher’s test. HCC: Hepatocarcinoma.
Mortality from COVID-19 was 5.7% (n = 31), with an incidence rate of 2.9 (95% confidence interval [CI]: 2-4.2) and a median survival of 18 days (IQR: 12-32). The only variable related to liver disease with statistically significant differences in mortality was cirrhosis decompensation (p < 0.005), as shown in Table 2.
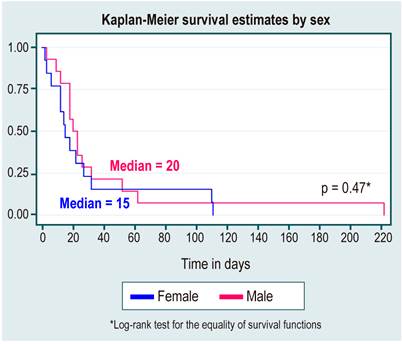
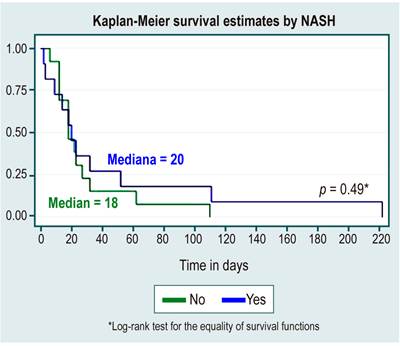
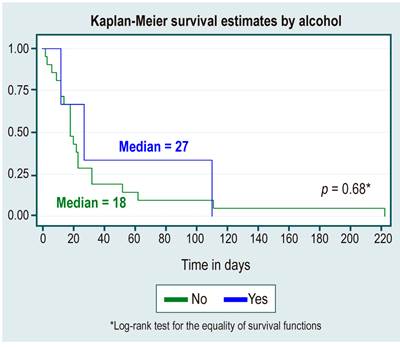
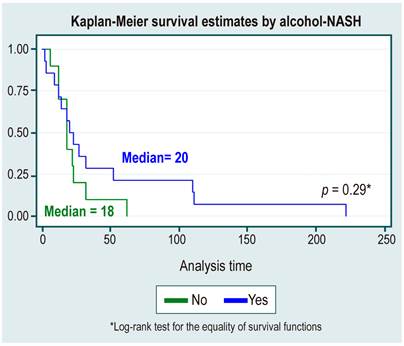
In the survival analysis, no statistically significant differences were found between survival curves by sex, cause of HCC, diagnosis or not of cirrhosis, etiology of cirrhosis due to NAFLD, or alcohol use, as shown in Table 3 and Figures 1,2,3,4 y5.
Tabla 3 Survival analysis
| Incidence rate * 100 (95%CI) | Median survival (days) | Percentile 25% - percentile 75% (days) | |
|---|---|---|---|
| Positive diagnosis | 2.9 (2 a 4.2) | 18 | 12-32 |
Discussion
We recognize the weaknesses of the study for being retrospective; however, until the writing of the manuscript, it is the first cohort in Colombia to describe the behavior of liver diseases in patients who become ill with COVID-19.
Similarities to other studies are found in which liver diseases have not posed an increased risk of SARS-CoV-2 infection. The descriptive studies published so far found that only a small number of patients with the condition (approximately 3%) have underlying chronic liver disease, and no statistically significant association between chronic liver disease and severity of COVID-19 or outcomes regarding mortality or severity of infection has been established11,13-15. The preceding is reflected in the incidence rate of 2.9, even considering that this study is based on those patients who already have chronic liver disease. Furthermore, it reveals that mortality in this series is probably related to other factors and in those patients having advanced disease with decompensated cirrhosis from the perspective of liver disease.
No statistically significant differences were found between the causes of liver disease that represent a higher risk of mortality from SARS-CoV-2 infection in this series, contrary to what has been described in other latitudes. It has been shown that chronic diseases such as hepatitis B and C occupy the first places12, even considering non-negligible rates of hepatitis C infection of 7% in the present study.
We ignore the transaminase behavior from the point of view of liver involvement, considering that baseline paraclinical tests are before infection. However, they show a slight elevation of ALT and AST. Other studies5,11,12 show liver enzyme elevations with SARS-CoV-2.
Compared to global reports of patients with liver disease who develop COVID-19, such as SECURE-Cirrhosis and COVID-HEP. Mortality was similar at 13.8% for SECURE-Cirrhosis, but with a difference from COVID-HEP, in which 36.5% of deaths were found among patients with chronic liver disease and liver transplants16,17.
Only one report of a transplanted patient was found, leaving the door open for research in this group of patients, who already have a higher risk of mortality from any cause because they are immunosuppressed patients. Nonetheless, this reinforces the concept of protection with immunization since this group of patients has a lower immunogenic response (approximately 50%), unlike those immunocompetent with responses close to 94%18,19.
As it was a study conducted in an outpatient center, the primary liver disease was NAFLD associated with obesity and dyslipidemia, which until now has been briefly described in a few observational studies20,21. These found that mainly young patients and those with NAFLD have a higher risk of severe disease and a longer viral shedding time. However, fatty liver is frequently associated with other comorbidities such as diabetes or cardiovascular disease, which are also established risk factors for severe COVID-19 and could contribute to worse outcomes in these patients. Thus, there is a need for an adequate diagnosis of NAFLD to adequately define if it represents a cardiovascular risk factor in addition to chronic comorbidities, such as arterial hypertension or diabetes, once again reinforcing the concept of early vaccination in this population deemed a risk group.
Conditions of causality could not be established due to the characteristics of the study. Long-term observations are required to define the impact of liver diseases in severe cases of SARS-CoV-2 illness and determine potential therapeutic interventions in those patients with chronic liver diseases. Until now, there are discordant results as to whether or not they are a factor of more significant mortality from COVID-1922,23.
Conclusion
Despite being a retrospective study, it is probably the first cohort of patients with liver diseases affected by SARS-CoV-2. Similarities and differences with other studies are found, but prospective studies are needed to assess the impact of chronic liver diseases on SARS-CoV-2 infection.











 text in
text in