Case report
Giant pancreatic pseudocyst drainage by endoscopic cystogastrostomy: Case report
1MD. Gastroenterology Specialist, San Rafael University Hospital, Tunja, Colombia.
2MD. Epidemiology Specialist, San Rafael University Hospital, Tunja, Colombia.
Abstract
Introduction:
The pancreatic pseudocyst is one of the late local complications of acute pancreatitis. For managing a giant pancreatic pseudocyst, there are multiple strategies.
Aim:
To present the case of a patient with a giant pancreatic pseudocyst managed by endoscopic cystogastrostomy.
Clinical case:
A 41-year-old woman developed a giant pancreatic pseudocyst as a complication of acute pancreatitis that was managed by endoscopic cystogastrostomy without endoscopic ultrasound guidance, with good evolution.
Conclusions:
Endoscopic cystogastrostomy, with or without the help of ultrasound endoscopy or lumen-apposing metal stent (LAMS), is a viable, safe, effective, and economical therapeutic option for selected patients with a giant pancreatic pseudocyst.
Keywords: Pancreatic pseudocyst; cystogastrostomy; endoscopy; pancreatitis; complications
Resumen
Introducción:
el pseudoquiste pancreático es una de las complicaciones locales tardías de la pancreatitis aguda. Para el manejo del pseudoquiste pancreático gigante existen múltiples estrategias.
Objetivo:
presentar el caso de una paciente con pseudoquiste pancreático gigante manejado mediante cistogastrostomía endoscópica.
Caso clínico:
mujer de 41 años que desarrolló un pseudoquiste pancreático gigante como complicación de una pancreatitis aguda y se manejó mediante cistogastrostomía endoscópica sin guía ecoendoscópica, con una adecuada evolución.
Conclusiones:
la cistogastrostomía endoscópica, con la ayuda o no de ecoendoscopia ni stent de aposición luminal (LAMS), es una opción terapéutica viable, segura, efectiva y económica para pacientes seleccionados con pseudoquiste pancreático gigante.
Palabras clave: Pseudoquiste pancreático; cistogastrostomía; endoscopia; pancreatitis; complicaciones
Introduction
The pancreatic pseudocyst is a liquid collection of debris, surrounded by fibers and inflammatory tissue and lacking epithelial coverage. It can be found partially or totally within the pancreatic parenchyma1 and persists for more than six weeks after the condition onset2. It arises as a complication of acute3 or chronic4 pancreatitis, trauma5, or other processes that affect the pancreatic duct6.
The symptoms are usually abdominal pain, vomiting, nausea, and weight loss7. Diagnosis is made by transabdominal ultrasound, tomography, magnetic resonance, or endoscopy, the latter being the method of choice8.
This manuscript intends to present the case of a woman with a pancreatic pseudocyst secondary to acute pancreatitis of unclear etiology and its evolution after endoscopic cystogastrostomy.
Clinical case
A 41-year-old woman from western Boyacá had a one-month history of severe epigastric pain and vomiting, exacerbated in the last 15 days. With no history of alcohol consumption, she attended the regional hospital. Abdominal tomography showed a pancreatic pseudocyst measuring 88 x 74 x 68 mm. She was administered analgesics and discharged; however, she was re-admitted due to the persistence of pain. The control abdominal tomography revealed continuance of the pancreatic pseudocyst in contact with the posterior wall of the lesser curvature of the stomach, for which she was referred to tertiary care.
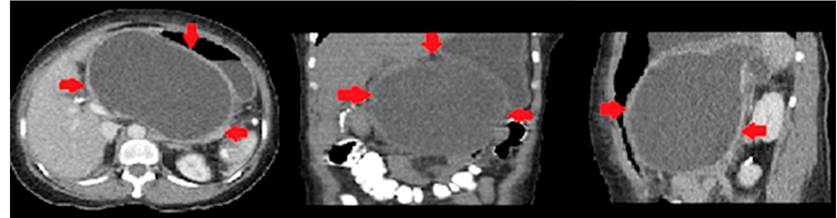
On physical examination upon admission to our institution, the patient presented with a distended abdomen, pain, a palpable mass in the epigastrium, and no jaundice. The paraclinical tests showed a complete blood count without leukocytosis, hyponatremia, mild hypochloremia and hypokalemia, no metabolic acidemia, slightly increased lactate, mild hyperbilirubinemia, at the expense of direct hyperbilirubinemia, and mild amylasemia. Possible acute pancreatitis was considered. Therefore, we decided to perform a contrast-enhanced computed tomography of the abdomen, which reported a pseudocyst with an approximate volume of 1,460 mL in contact with the posterior gastric wall (Figure 1).
Upper GI endoscopy was performed, finding compression of the posterior stomach wall. Due to the impossibility of referral to a center with endoscopic ultrasound availability, we decided to drain it with endoscopic cystogastrostomy at the end of the eighth week.
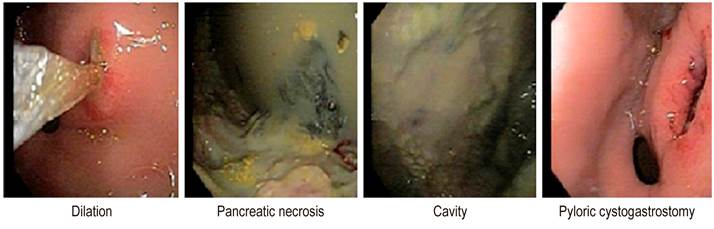
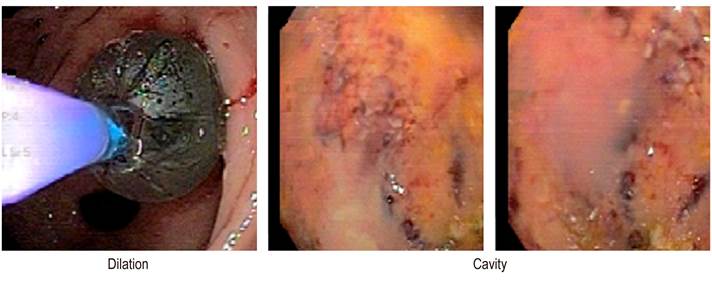
The posterior stomach wall was incised near the antrum with an output of necrotic and pancreatic material of approximately 1,500 mL. The cystogastrostomy was dilated with a balloon of up to 18 mm in diameter in operating rooms (Figure 2).
Endoscopic cholangiography was taken at the same surgical time, ruling out choledocholithiasis. Pancreatography was performed, finding dilatation of the pancreatic duct without disruption, followed by a pancreatic and biliary papillotomy.
On the third day after the primary intervention, the patient had signs of systemic infection, fever (39.2), leukocytosis, and positive procalcitonin (31.3). In the culture report of the pseudocyst fluid, there was E. coli growth. Piperacillin/tazobactam was administered for eight days, and endoscopic necrosectomies were performed under sedation every 24 to 48 hours (Figure 3).
On the third day after antibiotic treatment, normalization of acute phase reactants and improved liver function tests were observed. Thirteen washes were performed through endoscopic cystogastrostomy, achieving clinical and paraclinical improvement with discharge on day 45. Follow-up was conducted for six months, noting good evolution with no abdominal pain or relapses, no functional limitation, and weight gain.
Discussion
The incidence of pancreatic pseudocyst is low (1 per 100,000 adults per year), while the prevalence is 6%-18.5%. According to the etiology, the pseudocyst appears in 20%-40% of cases of chronic pancreatitis, 70%-78% is associated with pancreatitis of alcoholic etiology, followed by chronic idiopathic pancreatitis in 6%-16% and chronic pancreatitis of biliary etiology in 6%-8%9. According to the Atlanta classification10, giant pancreatic pseudocysts can occur after acute pancreatitis and have a diameter greater than 10 cm11.
There are few observations in the literature on the management of giant pancreatic pseudocysts. One study found that expectant management is associated with higher morbidity and mortality compared to small pseudocysts, suggesting that early external drainage before clinical deterioration could be beneficial12,13.
As described in the literature, the pseudocyst treatment was performed after the fourth week of onset in this case14. The choice of treatment for giant pancreatic pseudocysts is controversial. It includes observation, endoscopic drainage guided or not by endoscopic ultrasound, percutaneous drainage, and surgical interventions15. Currently, the endoscopic approach is preferred, as it is less invasive; it has a success rate of up to 95%16. If associated with ultrasound endoscopy, it has fewer complications and is more cost-effective17, avoids external drainage (success rate of 98.3%, with recurrence of 2.5%16, and has a high long-term success rate. Nonetheless, the form of drainage, whether transmural or transpapillary, is still divergent; when comparing them, they do not provide a more significant benefit in the treatment outcome.
In the guidelines of the Society of Endoscopic Gastroenterology of India18, the use of the pigtail catheter is mentioned as a drainage method; however, in the case of pseudocysts with necrotic content, they recommend management with a metallic stent. The endoscopic approach showed advantages; recovery was adequate and without complications, despite lacking the help of endoscopy or LAMS, which are the techniques of choice for similar cases19.
Conclusion
Endoscopic cystogastrostomy guided or not with endoscopic ultrasound and without luminal apposition stent is a viable, safe, effective, and economical therapeutic option for selected patients with a giant pancreatic pseudocyst.
References
1. Agalianos C, Passas I, Sideris I, Davides D, Dervenis C. Review of management options for pancreatic pseudocysts. Transl Gastroenterol Hepatol. 2018;3(3):18. https://doi.org/10.21037/tgh.2018.03.03
[ Links ]
2. Gómez M, Otero W. Manejo del seudoquiste pancreático y la necrosis infectada. En: Aponte DM (editor). Tratado de pancreatología. Bogotá, Colombia: Editorial Panamericana Formas e Impresos. 2015. p. 107.
[ Links ]
3. Memiş A, Parildar M. Interventional radiological treatment in complications of pancreatitis. Eur J Radiol. 2002;43(3):219-28. https://doi.org/10.1016/s0720-048x(02)00157-2
[ Links ]
4. Radojkovic M, Kovacevic P, Radojkovic D. Pancreatic pseudocyst with spontaneous cutaneous fistulization. Medicine (Baltimore). 2018;97(35):e12051. https://doi.org/10.1097/MD.0000000000012051
[ Links ]
5. Aghdassi AA, Mayerle J, Kraft M, Sielenkämper AW, Heidecke CD, Lerch MM. Pancreatic pseudocysts - When and how to treat? HPB (Oxford). 2006;8(6):432-41. https://doi.org/10.1080/13651820600748012
[ Links ]
6. Gómez-Zuleta M, Lúquez-Mindiola A, Ruíz-Morales O. Drenaje de pseudoquiste pancreático guiado por ecoendoscopia sin fluoroscopia: serie de casos. Rev Col Gastroenterol. 2017;32(2):160-5. http://dx.doi.org/10.22516/25007440.143
[ Links ]
7. Zárate-Suárez L, Mendoza-Saavedra J, Tovar-Fierro G, Arenas-Pinzón M. Drenaje transgástrico de pseudoquiste pancreático en paciente pediátrico. Rev Col Gastroenterol. 2018;33(2):161-5. http://dx.doi.org/10.22516/25007440.147
[ Links ]
8. Saluja SS, Srivastava S, Govind SH, Dahale A, Sharma BC, Mishra PK. Endoscopic cystogastrostomy versus surgical cystogastrostomy in the management of acute pancreatic pseudocysts. J Minim Access Surg. 2020;16(2):126-31. https://doi.org/10.4103/jmas.JMAS_109_18
[ Links ]
9. Khanna AK, Tiwary SK, Kumar P. Pancreatic pseudocyst: Therapeutic dilemma. Int J Inflam. 2012;2012:279476. https://doi:10.1155/2012/279476
[ Links ]
10. Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993 May;128(5):586-90. https://doi.org/10.1001/archsurg.1993.01420170122019
[ Links ]
11. Udeshika WAE, Herath HMMTB, Dassanayake SUB, Pahalagamage SP, Kulatunga A. A case report of giant pancreatic pseudocyst following acute pancreatitis: Experience with endoscopic internal drainage. BMC Res Notes. 2018;11(1):262. https://doi.org/10.1186/s13104-018-3375-9
[ Links ]
12. Behrman SW, Melvin WS, Ellison EC. Pancreatic pseudocysts following acute pancreatitis. Am J Surg. 1996;172(3):228-31. https://doi.org/10.1016/s0002-9610(96)00157-2
[ Links ]
13. Oblizajek N, Takahashi N, Agayeva S, Bazerbachi F, Chandrasekhara V, Levy M, et al. Outcomes of early endoscopic intervention for pancreatic necrotic collections: A matched case-control study. Gastrointest Endosc. 2020;91(6):1303-9. https://doi.org/10.1016/j.gie.2020.01.017
[ Links ]
14. Gurakar M, Faghih M, Singh VK. Endoscopic intervention in pancreatitis: Perspectives from a gastroenterologist. Abdom Radiol (NY). 2020;45(5):1308-15. https://doi.org/10.1007/s00261-019-02314-7
[ Links ]
15. Braghetto-Miranda I, Jiménez-Yuri R, Korn O, Arellano L. Manejo quirúrgico de pseudoquiste pancreático gigante: caso clínico. Rev Cirugía. 2021;73(2):217-21. https://dx.doi.org/10.35687/s2452-454920210021010
[ Links ]
16. Misra D, Sood T. Pancreatic pseudocyst. Treasure Island (FL): StatPearls Publishing. 2021.
[ Links ]
17. Tan JH, Chin W, Shaikh AL, Zheng S. Pancreatic pseudocyst: Dilemma of its recent management (Review). Exp Ther Med. 2021;21(2):159. https://doi.org/10.3892/etm.2020.9590
[ Links ]
18. Shah R, Basha J, Rana S, Jagannath S, Rai P, Dhar S, et al. Endoscopic management of pancreatic fluid collections: Guidelines of Society of Gastrointestinal Endoscopy of India and Indian EUS Club. J Digest Endosc. 2021;12(01):003-010. https://doi.org/ 10.1055/s-0041-1728956
[ Links ]
19. Irisawa A, Miyoshi H, Itoi T, Ryozawa S, Kida M, Inui K. Recent innovations in therapeutic endoscopy for pancreatobiliary diseases. Dig Endosc. 2020;32(3):309-15. https://doi.org/10.1111/den.13473
[ Links ]











 text in
text in