Clinical case
A 16-year-old male patient, from Boyacá, has a history of a syncopal episode associated with emesis and an incidental finding of iron deficiency anemia, hypochromia, and microcytosis without a clear cause, initially treated with polymaltosed iron folate and folic acid. Within his controls with pediatric hematology, refractoriness to treatment was documented, so he was referred to gastroenterology, where a first endoscopy of the upper digestive tract was performed. The initial biopsies showed non-atrophic chronic gastritis antrum of a follicular pattern at the level of the with mild activity, associated with Helicobacter pylori infection, for which tetraconjugate management was indicated for 14 days.
After eradication of the infection, ferrous sulfate 300 mg and ascorbic acid 500 mg twice daily were started to manage the iron deficiency; the patient returned to control due to the persistence of significant drowsiness and fatigue. His paraclinical tests (Table 1) showed the correction of the volumes but the persistence of iron deficiency despite an adequate intake.
Table 1 Laboratory test records
| Reference values | Control # 1 | Control # 2 | Control # 3 | Control # 4 | Control # 5 | Control # 6 | |
|---|---|---|---|---|---|---|---|
| Leukocytes (uL) | 4500-11 500 | 8000 | 6800 | 6500 | 5700 | 7690 | |
| Neutrophils (uL) | 1400-6500 | 4100 | 4000 | 3600 | 2600 | 4940 | |
| Lymphocytes (uL) | 1200-3400 | 2800 | 1900 | 2100 | 2200 | 2130 | |
| Eosinophils (uL) | 0-700 | 300 | 400 | 300 | 400 | 200 | |
| Hemoglobin (g/dL) | 14-18 | 13.8 | 14 | 15.5 | 16.1 | 16.3 | 18.1 |
| Hematocrit (%) | 45-54 | 40.7 | 44.50 | 47.90 | 47.70 | 49.40 | 54.20 |
| Mean corpuscular volume (fL) | 80-100 | 73.6 | 82.4 | 83.5 | 87.20 | 94.10 | |
| Mean corpuscular hemoglobin (pg) | 25.4-34.6 | 23.8 | 26.7 | 18.1 | 28.80 | 31.40 | |
| Distribution width (%) | 11.5-18 | 18 | 14.90 | 16 | 16.8 | 12.5 | |
| Platelets (10^3/uL) | 150-450 | 339 | 315 | 343 | 325 | 307 | |
| Ferritin (ng/mL) | 23.9-336.2 | 3.74 | 9.2 | 18 | 116.4 | 60 | |
| Parietal cell antibodies | Positive >1/40 | 1/640 | |||||
| Intrinsic factor antibodies | Negative (-) | (-) | |||||
| Gastrin (pg/mL) | 13-115 | 274 |
Source: Prepared by the authors.
In the fourth control, serological results compatible with the diagnosis of autoimmune gastritis were obtained. After the management of iron deficiency in the last controls, a normalization of hemoglobin and iron deposits was found.
A new esophagogastroduodenoscopy was performed with a body, antral and esophageal mucosa biopsy, finding chronic atrophic gastritis with extensive pseudopyloric metaplasia (antralization) and moderate activity in the body mucosa (oxyntic), associated with linear and nodular hyperplasia of neuroendocrine cells (enterochromaffin). The latter was highlighted by the immunohistochemical study for chromogranin (Figure 1). Meanwhile, in the antral mucosa, mild non-atrophic chronic gastritis was observed, with residual lymphoid aggregates, no acute inflammatory activit,y and evidence of bacilli with Helicobacter pylori morphology. The findings of chronic atrophic gastritis restricted to the oxyntic mucosa with hyperplasia of neuroendocrine cells (enterochromaffin) suggest autoimmune gastritis. Additionally, infection compatible with Candida sp. at the esophageal level was documented. Consequently, the autoimmune profile was completed based on the histological findings, the medical management was adjusted, and finally, the diagnosis of autoimmune gastritis was made given the presence of positive anti-parietal cell antibodies.
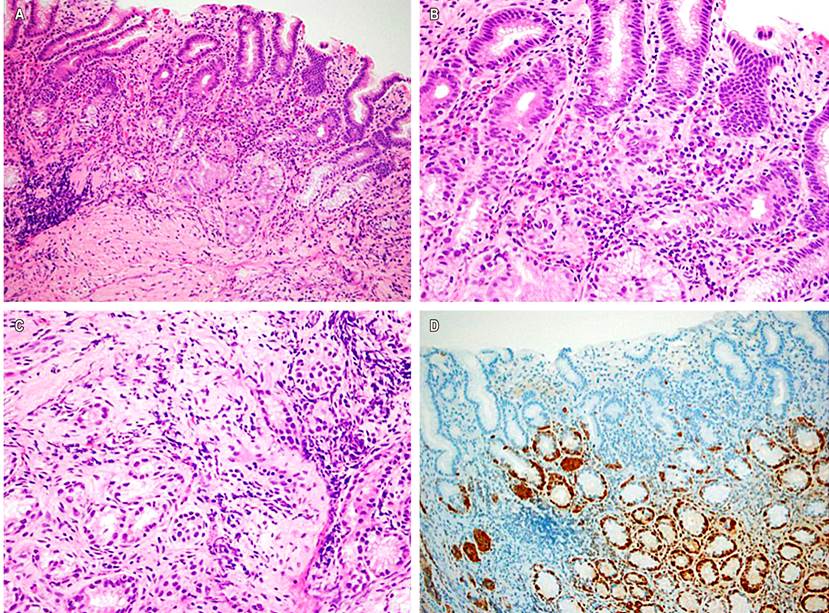
Figure 1 Body gastric mucosal biopsy. A. Oxyntic-type mucosa with loss of parietal cells and centralization of the mucosa, accompanied by a diffuse inflammatory infiltrate that compromises the entire thickness of the mucosa (Hematoxylin-eosin 100X). B. Lymphoplasmacytic inflammation in the lamina propria, rich in plasma cells and accompanied by eosinophils, noting permeation of the glands. C. Micronodules of neuroendocrine cells in the mucosa (Hematoxylin-eosin 400X). D. Immunohistochemical study for chromogranin confirmed a linear and nodular proliferation of neuroendocrine cells (100x). Source: Owned by the authors.
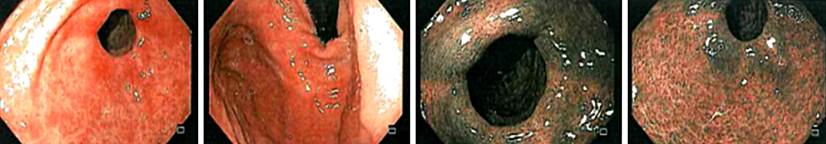
In subsequent controls, replacement with intravenous iron was necessary once. Currently, the patient does not require medical management. The last control esophagogastroduodenoscopy (Figure 2) showed corporoantral erythematous gastritis, whose biopsies showed a decrease in gastrin marking, which corresponds to the already known diagnosis, with paraclinical tests within normal limits.
Discussion
Autoimmune gastritis is an underdiagnosed disease worldwide, especially in childhood, due to its asymptomatic nature1,2, on the one hand, and the early treatment given to hematological alterations once identified without first investigating their etiological diagnosis, on the other3.
Currently, autoimmune gastritis represents about 10% of gastritis cases in the adult population1. However, these estimates are not apparent in the pediatric population, where the average age of diagnosis is 12.3 years, with refractory iron deficiency anemia being the most common manifestation2, as in our case.
Autoimmune gastritis is a chronic inflammatory disease that selectively affects the gastric body and fundus, particularly preserving the antrum4. It is characterized by antibodies against the proton pump H/K ATPase and, to a lesser extent, against intrinsic factors1,2. Autoimmune gastritis is known to cause pernicious anemia in older adults, with the classic manifestation of megaloblastic anemia and cyanocobalamin deficiency. Nonetheless, recent studies have linked iron deficiency anemia to this entity in much earlier stages, even in the pediatric population1,2.
Currently, the etiopathogenesis of autoimmune gastritis is unknown. It is believed that it results from the interaction of genetic, hormonal, and environmental factors with still undefined defects in the immune response3,5; however, recent studies speak of the inappropriate activation of regulatory T cells1. Moreover, the association between autoimmune gastritis and Helicobacter pylori infection has been reviewed, finding molecular mimicry between bacterial antigens and proton pump receptors in parietal cells4. Some studies have shown the stimulation of T cells against parietal cells after infection, assigning it a role in the pathogenesis of autoimmune gastritis3.
At a functional level, the involvement of the parietal cells, which produce intrinsic factors, is responsible for the malabsorption of cyanocobalamin and the subsequent development of megaloblastic anemia. Similarly, specific antibodies against intrinsic factors may trigger the same clinical manifestations4.
Additionally, the secretion of hydrochloric acid3 is compromised with a subsequent rise in gastric pH, affecting the solubilization and reduction of iron, processes that require an acidic environment to be effective and that, when failed, facilitate the development of iron deficiency4,6. Similarly, an alkaline climate favors the colonization of the stomach by different microorganisms, as occurred in the case presented, which perpetuates a local inflammatory response3. In response to achlorhydria, there is a gastrin hypersecretory response, which favors the development of enterochromaffin cell hyperplasia and neuroendocrine tumors3,4.
Regarding the clinical manifestations of the disease, autoimmune gastritis is usually silent until a significant degree of glandular atrophy is reached. Then it can present with weakness, pallor, and other nonspecific symptoms secondary to anemia4,5, as in our patient, who started with episodes of syncope. The age of occurrence is usually variable and correlates with hematological manifestations7. Young patients typically have iron deficiency anemia refractory to treatment, while older adults show symptoms related to megaloblastic anemia secondary to chronic consumption of cyanocobalamin reserves1-4,6.
The diagnosis is based on antibodies against parietal cells, intrinsic factors, or H/K ATPase3,4. A positive serology not only supports the diagnosis but also informs about the hematological involvement and the degree of atrophy4. Currently, different gastrointestinal panels in the world allow the identification of fundic atrophy; these include biomarkers such as levels of pepsinogen I and II, the ratio between these two, and gastrin levels4,5.
Furthermore, it is crucial to carry out endoscopic studies in which focal gastritis at the body level, a loss of the anatomical folds characteristic of this region, and the presence of pseudopolyps can be viewed1,3,7. A representative sample must be available to make an adequate histopathological diagnosis, considering the topographic location due to the focal nature of autoimmune gastritis and the need to distinguish Helicobacter pylori infection1-3.
The histopathological spectrum of autoimmune gastritis is vast and includes four phases, sometimes overlapping. The first phase consists of a lymphoplasmacytic infiltrate in the lamina propria, which is usually diffuse and accentuated at the base of the mucosa. The second phase is characterized by the destruction of the oxyntic glands with a consequent pseudopyloric metaplasia (“antralization of the mucosa”), in addition to diffuse lymphoplasmacytic infiltrate in the lamina propria. The third phase comprises progressive destruction of the oxyntic glands accompanied by intestinal metaplasia or pancreatic acinar metaplasia. The final step is a total replacement of the oxyntic glands by a metaplastic epithelium and the absence of a prominent inflammatory component5.
Another characteristic finding of autoimmune gastritis is linear and nodular hyperplasia of enterochromaffin cells in response to achlorhydria, leading to the development of type 1 neuroendocrine tumors5.
Follow-up should be conducted with markers of atrophy, complete blood count, and screening for autoimmune diseases4. As with other conditions of this nature, pre-existing or family history is associated with a higher risk of suffering from other autoimmune disorders, most commonly thyroiditis and type 1 diabetes mellitus1,3,4. There was no family history of autoimmunity in the case presented, and evaluation of other autoimmune diseases was negative.
The management of these patients is aimed at controlling hematological manifestations and preventing their progression3. Iron deficiency anemia is corrected by supplementation of the element according to baseline requirements1,2 and, in the case of refractoriness, defined as failure to respond to oral iron treatment for at least two months6, the administration of intravenous iron is chosen to replenish its deposits6. In advanced cases with pernicious anemia, management focuses on controlling cyanocobalamin reserves2,3.
So far, there is no certainty about the prognosis of these patients. Studies show a decrease in the progression of the disease but not a reversal of the changes established2. Additionally, as it is a chronic inflammatory process that involves changes in the epithelial lining, the risk of gastric cancer in these patients should not be neglected7. An endoscopic control is recommended every five years to monitor for preneoplastic lesions and evaluate their progression1,2.
Conclusion
Autoimmune gastritis must be recognized as a disease that occurs in the pediatric population. Although its symptoms are nonspecific, the incidental finding of iron deficiency anemia refractory to treatment should suggest its diagnosis. Early identification allows treatment and implementing strategies to prevent its progression. Currently, treatment focuses on the control of identified deficiency anemia, in addition to endoscopic follow-up, for the early detection of preneoplastic lesions and malignancy in the region in adulthood.











 texto en
texto en