Introduction
Graft-versus-host disease (GVHD) is a frequent complication of allogeneic stem cell transplantation caused by an immune response of donor lymphocytes against the recipient patient1. The incidence of GVHD in this transplant is estimated to be as high as 39%-59%, with first-year mortality of 31%2. The skin, the gastrointestinal tract (GIT), and the liver are the main organs affected. The GIT is affected in up to 74% of cases and may be exclusively compromised in 17% of cases; 67% of cases with digestive compromise show diffuse involvement of both the upper and lower GIT3. We present three cases of patients treated at the Pablo Tobón Uribe Hospital (HPTU) in Medellín with suspected GVHD and gastrointestinal involvement and their diagnostic approach.
Case 1
A 26-year-old male patient diagnosed with B-cell acute lymphoblastic leukemia received induction therapy with the high-risk PETHEMA-LAL protocol and three consolidation cycles with PETHEMA. Subsequently, he had a systemic relapse with compromise in the central nervous system. High-intensity chemotherapy with IDA-FLAG, cytarabine, and intrathecal therapy was administered, achieving morphological remission with residual marrow disease, for which he required new chemotherapy for bone marrow transplantation (BMT). Haploidentical transplantation of hematopoietic progenitors was performed with his mother as a donor. He was admitted on day 24 after the transplant due to acute diarrhea, generalized mucocutaneous jaundice, and diffuse erythematous macular exanthema with scaling. Paraclinical tests revealed pancytopenia and an altered liver profile with a mixed pattern (hepatocellular and cholestatic). Given the suspicion of GVHD, empirical management was started with high doses of methylprednisolone, requesting endoscopic studies. Esophagogastroduodenoscopy (EGD) revealed relevant findings in the mucosa of the first and second portions of the duodenum with focal areas of patchy atrophy and absence of villi, with no inflammatory changes and normal gastric mucosa (Figure 1).
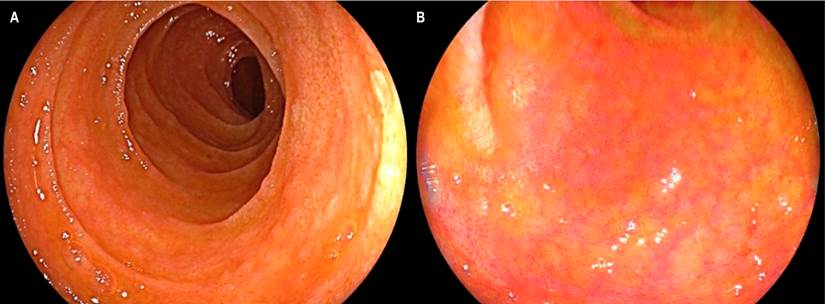
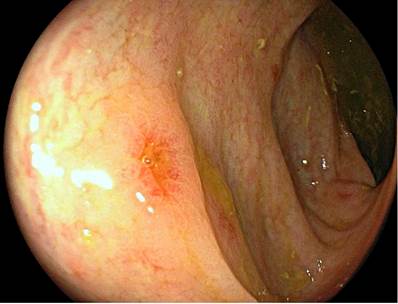
Figure 1 Compromise due to GVHD in the duodenal mucosa. A. Endoscopic view with a white light showing a reduction in the size and thickness of the duodenal folds, with an atrophic-looking mucosa. B. View with LCI (linked-color imaging) chromoendoscopy in which thinning of the mucosa was found, with signs of atrophy and shortening of villi.
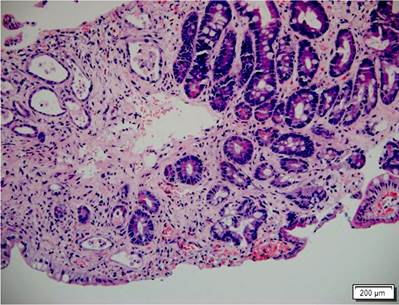
The ileum was accessed through a colonoscopy, finding a diffuse atrophy of the mucosa, absence of villi, and marked friability with easy bleeding and sloughing on rubbing, moderate bleeding, and even the formation of small submucosal hematomas when taking biopsies (Figure 2). The colonic mucosa was normal. The histopathological study of the biopsies of the duodenum and the ileum confirmed the acute grade 4/4 GVHD (Figure 3). The patient’s evolution was torpid with persistent diarrhea, high fecal output, digestive bleeding, hydro electrolytic disorders difficult to manage, multiple transfusions of blood components, and development of bacteremia due to Klebsiella pneumonia, and hypoxemic respiratory failure. He presented with acute, severe GVHD refractory to steroids, with poor prognosis criteria, whose outcome was death 56 days after hospitalization.
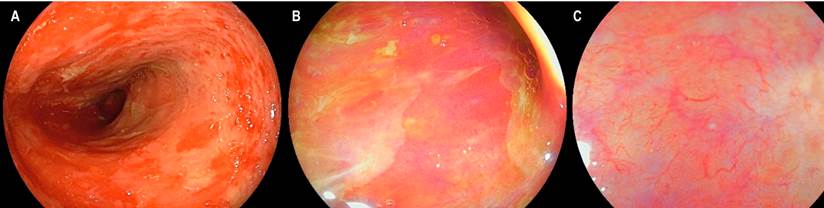
Figure 2 Compromise due to GVHD in the terminal ileum. A. White light endoscopic view demonstrating patchy sloughing of the ileal mucosa, atrophy, and marked friability. B. LCI view that shows an area of denudation without villi, findings confirmed in C when evaluated by LCI with magnification.
Case 2
We present the case of a 17-year-old male patient diagnosed with T-lymphoblastic lymphoma, who initially received induction and reinduction therapy with the GRAALL Lysa protocol (cytarabine + idarubicin), achieving remission of his disease. He underwent haploidentical hematopoietic stem cell transplantation with his father as the donor. He developed a diffuse macular rash, predominantly in the extremities during the transplant. Skin biopsy confirmed the diagnosis of acute GVHD with grade 3/4 skin involvement. On day 32 after the transplant, he presented with fever, abdominal pain in the hypogastrium and both iliac fossae, and liquid stools without mucus or blood. Computed axial tomography (CAT) of the abdomen documented inflammatory changes in the ileocecal region and signs of terminal ileitis; thus, endoscopic studies were requested. The EGD revealed flat patchy erythema in the stomach without inflammatory changes in the duodenum. Colonoscopy revealed congestive mucosa of the ileum with multiple superficial, irregular ulcers with flat edges and a smooth surface. Furthermore, in all the colonic tracts, including the rectum, small punctate ulcers with fibrin in the center and flat congestive edges were observed (Figure 4). The histopathological study confirmed the diagnosis of acute GVHD in the ileum grade 3/4, right colon 1/4, left colon 3/4, and rectum 4/4 (Figures 5 and 6). Immunohistochemistry (IHC) on these samples was negative for cytomegalovirus (CMV).
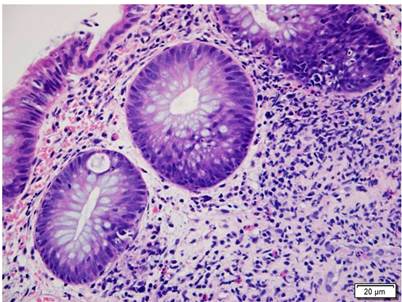
Figure 5 Colon compromised by GVHD. Colon histopathology with H&E (400 X). Shows crypt damage with basal apoptotic bodies and mixed inflammatory infiltrate in the lamina propria around the crypts. GVHD grade 1.
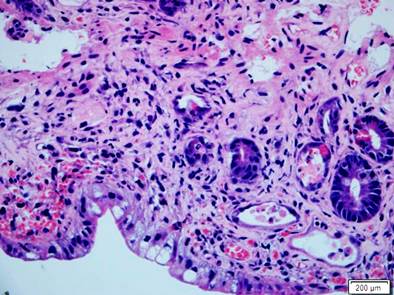
Figure 6 Small intestine compromised by GVHD. Histopathology of the small intestine with H&E (400 X). An apparent decrease in the number of crypts with marked mucin depletion, presence of basal apoptotic bodies, and adjacent mixed inflammatory infiltrate stand out. Severe GVHD.
Initially, management was provided with high doses of methylprednisolone with partial response. Thus, around day 15 of the disease, we decided to carry out endoscopic control with EGD, not showing any relevant findings, and colonoscopy, which even showed a worsening of the findings in the ileum with sloughing of the mucosa, diffuse atrophy without villi, and no changes on the ulcerations in the colon. These manifestations correlated with the progression of acute GVHD to grade 4/4 but was also positive for CMV on IHC in biopsies. Management with ganciclovir was started, followed by ruxolitinib for second-line management of GVHD. Nevertheless, the clinical evolution was towards deterioration, with worsening liver involvement due to GVHD and progressive anemia due to digestive bleeding. Rescue therapy with infliximab was even started without any response, which finally resulted in the death of the patient after 58 days of hospitalization.
Case 3
A 41-year-old female patient diagnosed with acute myeloid leukemia and myelomonocytic maturation underwent a hematopoietic stem cell transplant after induction with a HIDAC chemotherapy scheme (high-dose cytarabine) plus midostaurin due to refractoriness to the first induction with a 7 x 3 scheme with cytarabine and idarubicin. Haploidentical transplantation of hematopoietic progenitors was performed with her brother as a donor. She was admitted on day 34 post-transplant with a two-week clinical picture consisting of diarrheal stools without mucus or blood, associated with crampy abdominal pain, nausea, and hyporexia. The clinical condition was generally acceptable, with no shock or inflammatory response signs. The paraclinical tests noted anemia of standard volumes and normal hepatic and renal functions. Given the suspicion of GVHD, an EGD were performed without pathological changes and a colonoscopy with evidence of mucosa of the left colon, sigmoid, and rectum with focal areas of congestion and aphthoid microerosions. Biopsies suggested acute GVHD in the duodenum grade 1/4 and in the rectum and sigmoid 3/4.
Given the acuteness of the condition, infectious colitis and positive Clostridium difficile toxin fecal colitis were suspected, with no other clinical signs suggestive of GVHD. Management included oral vancomycin for ten days with an adequate initial response, although with a subsequent recurrence of symptoms; therefore, she was hospitalized two months later. A high and low endoscopy control was performed, finding only ulcer scars in the colon with biopsies suggestive of GVHD grade 1/4 with negative IHC for CMV. Due to the positive serum viral load for CMV, oral valganciclovir was provided with improved digestive symptoms. Finally, it was not clear whether the patient’s digestive manifestations corresponded to GVHD or changes due to multiple infectious processes.
Discussion
GVHD is a frequent complication in patients undergoing allogeneic stem cell transplantation. However, cases have also been reported in patients undergoing autologous stem cell transplantation, solid organ transplantation, or after blood transfusions1. There are two variants of the disease: acute and chronic. Previously, a distinction was made between both types from the onset of the complication (acute if within the first 100 days of transplant). However, the 2005 consensus, as ratified by the 2014 National Institutes of Health (NIH) consensus, established that the difference between both types is based on clinical criteria according to the organs compromised instead of a specific time window4.
GVHD is a multisystemic disorder: The main organs affected are the skin, GIT, and liver5, and digestive involvement is the most difficult to manage and the one associated with the worst prognosis since it represents the leading cause of mortality related to GVHD4-7. The main risk factors for acute GVHD include major histocompatibility complex (MCHC) disparity, chronic myeloid leukemia, patient and donor age, history of acute GVHD, graft procurement method, and mismatch of sex, mainly when the recipient is male, and the donor is female6,8.
The pathophysiology of GVHD is not entirely elucidated; tissue damage is considered to be mainly mediated by donor T cells and proinflammatory cytokines6. It begins with the first phase of tissue injury, resulting from the myeloablative regimen with chemoradiation therapy prior to the donor graft, with subsequent production of proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin (IL) 1, 2, and 6, among others. They increase the expression of cell adhesion molecules, costimulatory molecules, and CMHC antigens, activating antigen-presenting cells9. Subsequently, there is activation, proliferation, and differentiation of the donor’s T cells towards the Th1, cytotoxic T, and Th17 subtypes, culminating in a cytotoxic effect with tissue damage1.
In the GIT, immune deregulation produces a disturbance in the intestinal epithelium, specifically in the stem, Paneth, and goblet cells6; in fact, histologic severity in intestinal GVHD is categorized according to the degree of crypt damage, and preservation of Paneth cells in duodenal biopsies is inversely correlated with disease severity, response to treatment, and transplant-related mortality7. It has also been suggested that an imbalance in the intestinal microbiota may play a role in developing GVHD, as there is a relationship between the innate and adaptive immune systems and intestinal bacteria. Factors such as the myeloablative regimen prior to transplantation, broad-spectrum antibiotics, immunosuppressive drugs, and the addition of donor lymphocytes influence this imbalance6,7.
The diagnosis of the acute variant is clinical. The NIH consensus criteria subclassify it as classic, with typical symptoms given by the appearance of an erythematous maculopapular rash, cholestatic hepatitis, and GIT symptoms such as diarrhea, nausea, vomiting, and abdominal pain within the first 100 days of transplantation10, or as a persistent, recurrent or late-onset form when typical symptoms appear after 100 days4. The diagnosis can be confirmed with a histopathological study of the skin or GIT1. Acute GVHD can affect any segment of the GIT, usually in a patchy manner, and the manifestations in this system can be very subtle and nonspecific, posing a diagnostic challenge. In the oropharynx, it can exhibit mucositis that can be difficult to differentiate from that induced by myeloablative treatment, although the latter is expected to manifest within the first three weeks after the transplant and, afterward, could be explained by GVHD6.
Gastroduodenal involvement shows mild and insidious symptoms such as loss of appetite, early satiety, dyspepsia, and weight loss, which can progress to incessant emesis, epigastric pain, and digestive bleeding3. The large and small intestines can also be affected, and diarrhea is the initial manifestation, usually occurring two weeks after the transplant6. Diarrhea is usually of the secretory type and is up to several liters per day in severity; as the inflammatory process progresses, a loss of proteins can occur through the mucosa. It generates mucus and, finally, bloody stools in the context of complete denudation of the epithelium, particularly in the ileum9. Mucosal injury with protein loss can result in malabsorption and malnutrition6.
The chronic variant can have a more varied manifestation involving multiple organs, including the lungs, hepatobiliary system, musculoskeletal system, GIT, and skin1. The diagnosis is made by identifying pathognomonic signs and symptoms, which, if present, are sufficient for diagnosis, such as poikiloderma in the skin or stenosis in the GIT4. Lastly, a subtype of the chronic variant is also described, characterized by symptoms typical of the acute variant simultaneously; this subtype is known as overlap syndrome and carries a worse prognosis than the classic chronic variant10.
Endoscopic studies are a fundamental piece in the study of GVHD. Generally, they perform well and are safe, with low complication rates11. The GVHD manifestation varies depending on the extent and severity and can range from patchy mucosal areas with mild and superficial erythema to ulcerations with sloughing and complete mucosal denudation (Table 1). Endoscopic techniques with magnification favor the detection of subtle changes such as shortening and reduction of the number of villi in the duodenum and ileum12. From the histological point of view, the severity of the findings will be defined by the degree of cell apoptosis in the crypts, necrosis, or, as in endoscopy, the evidence of complete mucosal denudation (Table 2).
Table 1 Freiburg classification
| Grade | Endoscopic finding |
|---|---|
| 1 | Normal mucosa |
| 2 | Patchy erythema |
| 3 | Aphthoid lesions or focal erosion |
| 4 | Confluent erosions, ulceration, or mucosal denudation |
Modified from13.
Table 2 Severity classification
| Grade | Pathological finding |
|---|---|
| 1 | Increased apoptosis in the crypts |
| 2 | Apoptosis with cryptic abscesses |
| 3 | Individual crypt necrosis |
| 4 | Total mucosal denudation in areas |
Modified from6.
Given the broad spectrum of GIT manifestation, the performance of endoscopic studies in diagnosing GVHD is variable, with sensitivity and specificity of 34%-89% and 65%-79%, respectively, and an agreement between the endoscopy and histology as low as 38%13,14. These are also used to rule out other differential diagnoses such as mycophenolate enteritis and colitis, infection by germs such as CMV, C. difficile, or common enteric viruses (adenovirus, rotavirus, norovirus, among others). Particularly in the context of CMV, given the high infection rates in bone marrow transplant patients (up to 15%) and the importance of its diagnosis, it is always advisable to perform an IHC or polymerase chain reaction (PCR) study on biopsies in tissue. Similarly, infection by C. difficile can occur between 12% and 27% in this group of patients, which is why it should also be ruled out before considering the diagnosis of GVHD15. Therefore, biopsies are always recommended, both for healthy mucosa and for mucosa with inflammatory changes6.
The diagnostic yield of endoscopic studies with biopsies is variable: 67%-80% for EGD, 58%-80% for rectosigmoidoscopy (RSC), 83%-87% for colonoscopy, 87%-100% for ileocolonoscopy, and 92%-93% for EGD with RSC11,13. Several studies suggest that CRS with biopsies of the distal colon could be more accurate in diagnosing acute GVHD (82%-95%); therefore, it is considered the initial study of choice, in addition to its easy preparation and performance13. The guidelines of the American Society for Gastrointestinal Endoscopy (ASGE) to approach diarrhea endorse this recommendation and suggest performing EGD in cases where CRS does not provide a diagnosis or if upper digestive symptoms are present16. Another guide published by the same society for taking samples in endoscopy suggests two approaches: RSC with four biopsies of the rectum and left colon; if no diagnosis is obtained, add EGD with biopsies of the body, antrum, and duodenum (four in each segment), or ileocolonoscopy with four biopsies of the distal ileum, right, transverse, left, and rectosigmoid colon17. In cases where CMV is suspected, performing RSC alone could be insufficient; performing a complete colonoscopy would be ideal. When taking biopsies, areas with very severe inflammation should be avoided due to the difficulty in interpretation for the pathologist and to reduce the risk of hematomas (in patients with thrombocytopenia) and even perforation, particularly in the duodenum and ileum11. Similarly, prophylactic antibiotics are recommended in patients with a neutrophil count < 500 cells/µL.
Endoscopic findings can also predict response to steroid management. A recent study of 44 patients with acute GVHD, of whom 45% were considered steroid-resistant, found that macroscopic findings in the ileum, histological findings in the ileum and colon, and the presence of granulation tissue in biopsies were predictors of refractoriness to steroid therapy18. In patients with therapeutic failure in the first line of management, repeating endoscopic studies could be considered to reassess the stage of the disease, objectify the degree of response, and rule out differential diagnoses (up to 25% of patients with therapeutic failure could have an infection by CMV in the second endoscopic review)19. Finally, some small studies of patients with suspected GVHD and capsule endoscopy have found high sensitivity (100%) and negative predictive value (NPV). As in conventional studies, the findings are usually patchy and range from erythema to erosions or ulcerations20-22. Generally, its use is recommended in patients with a high suspicion of GVHD who cannot tolerate conventional studies due to their clinical condition.
The standard GVHD treatment is high-dose steroids, particularly methylprednisolone 1-2 mg/kg/day in divided doses or prednisolone (1 mg/kg/day). In colonic compromise due to GVHD, the use of budesonide MMX has been described6,23. The response to steroid therapy is variable; up to 31%-57% of patients may be steroid-resistant24. Oral intake should be discontinued in patients with severe digestive symptoms, providing parenteral nutrition. For upper digestive symptoms, proton pump inhibitors (PPIs) and sucralfate are suggested. The use of non-steroidal anti-inflammatory drugs (NSAIDs) and opiates, including loperamide, should be avoided due to the risk of bleeding and ileus. The use of octreotide and cholestyramine has been described to control diarrhea, but potential adverse effects and the risk of alteration in the absorption of other medications must be contemplated25,26.
Conclusion
In the three cases presented, we found a broad spectrum of GVHD manifestation, from mild symptoms and changes in the mucosa, due to erythema and erosions, to severe symptoms, such as bleeding, diarrhea difficult to manage, and extensive changes in the intestine with denudation of mucosa and severe atrophy in the small intestine. Magnification, particularly in the small intestine, was deemed helpful where there was a significant correlation between endoscopy and biopsies concerning atrophy. The risk of complications associated with endoscopic procedures should be considered, particularly bleeding and perforation. Although they did not occur in our cases, there were signs such as submucosal hematomas with biopsies that alerted us to the limitation in the number of samples to take. It is essential to rule out differential diagnoses, especially infectious ones, which could impact the patient’s outcomes. As we saw in our cases, repeating the endoscopic studies could be helpful in follow-up and excluding infection by opportunistic germs. Due to their clinical condition, adherence to the ASGE recommendations should be pondered in high-risk patients, only performing CSR for diagnosing GVHD, but bearing in mind that ileocolonoscopy allows for a complete diagnostic approach.











 texto en
texto en