Introduction
Helicobacter pylorus is a gram-negative bacterium that colonizes the gastrointestinal tract and has high implications, especially in the gastric tract, where it is considered a type I carcinogenic agent by the World Health Organization (WHO)1. Identifying it is critical for gastric carcinoma and public health-related issues since this cancer constitutes the fourth cause of morbidity and the second cause of mortality worldwide2. In Colombia, reports from the National Cancer Institute have flagged gastric cancer as the first cause of cancer mortality in men and the second in women3. H. pylori are characterized by their helical shape and flagella, crucial for its motility and successful colonization. The genes and proteins related to the microorganism’s motility in the gastric environment are well documented in the literature4.
As described by Dr. Pelayo Correa in his carcinogenesis model for intestinal-type gastric carcinoma, this pathogen activates in people genetically susceptible to the carcinogen cascade, beginning with an inflammatory response and generating active chronic gastritis that can evolve to atrophic gastritis, intestinal metaplasia, and dysplasia -which will end up causing cellular changes to carcinoma5. It is recommended to monitor the patient in preneoplastic conditions (atrophic gastritis and intestinal metaplasia) and preneoplastic lesions (dysplasia) every 2 to 3 years, performing several biopsies on the antrum and gastric body. Additionally, if documented in the patient’s histology, a treatment to eradicate H. pylori is recommended6. The Sydney system is currently the most widely used and recommended biopsy protocol. This system performs biopsies in the antrum (greater and lesser curvature, 2 cm from the pylorus), incisura angularis, and body (anterior and posterior wall, 8 cm from the cardia)7.
The microorganism migration theory associated with changes in gastric distribution is proposed given the hypothesis of changes in the microenvironment in the gastric mucosa affected by H. pylori, as in intestinal atrophy and metaplasia, and considering the motility characteristics of this pathogen. Additionally, current protocols do not involve taking biopsies in the gastric fundus and cardia8, which could lead to false negatives identifying H. pylori in these patients.
The objective of this study is to identify the distribution patterns of this pathogen in the presence of atrophy and intestinal metaplasia, which will establish a very close value to the actual prevalence and distribution of H. pylori in this context.
Materials and methods
Design and population
A descriptive observational cross-sectional study of adult patients diagnosed with intestinal atrophy or metaplasia in a tertiary care institution. Patients with proton pump inhibitor (PPI) use in the last six months, antibiotic use in the last 30 days, patients with a histological diagnosis of gastric cancer, patients with a history of gastric surgery, diagnosis of Zollinger-Ellison syndrome disease or gastric lymphoma, and patients who required emergency surgical management (bleeding, obstruction or perforation) were excluded.
Sampling and sample size
The sample size was calculated based on an estimated prevalence of a 43.3% H. pylori presence9, with an 8% absolute accuracy and a 95% confidence level for a minimum required sample size of 148 patients.
Procedure
All patients with atrophy and intestinal metaplasia history who entered the gastroenterology unit to undergo upper gastrointestinal endoscopy (for their pathology check or any upper gastrointestinal symptomatology) were given informed consent. In addition, this study’s objectives and the steps for its development were explained in detail in this consent. Likewise, each patient was given a questionnaire about PPI use and H. pylori eradication.
Once the clinical history of the previous diagnosis of intestinal atrophy or metaplasia was confirmed and, after ruling out gastrointestinal surgeries, or a history of gastric cancer -the upper endoscopy procedure and biopsy were performed as follows:
Antrum: 2 and 3 cm from the pylorus in the major and lesser curvature (2 biopsies)
Incisura angularis (1 biopsy)
Body: 8 cm from the cardia in the anterior and posterior wall (2 biopsies)
Stomach fundus
Subcardial region
These biopsies were sent to pathology. Once the results were available, information on the questionnaires and pathologies was collected in a data extraction tool, typed by the same operator, and a double review of the information was made.
Variables
Patient demographic and clinical information was collected, including pre-treatment information for underlying disease (atrophy or intestinal metaplasia) and current or previous use of PPIs. In addition, detailed information was collected on the histological diagnosis of the result of biopsies in the body and antrum, incisura angularis, subcardial region, and gastric fundus.
Analysis
The final database was consolidated in the Stata 13 statistic software, and a descriptive analysis of the information was performed. Categorical variables were described as absolute; relative frequencies and quantitative variables were described as measures of central tendency and dispersion depending on the data distribution. The Shapiro-Wilk test evaluated a p-value less than 0.05 for statistical significance. An exploratory bivariate analysis was performed comparing the type of initial diagnosis and diagnosis according to the anatomical location.
Results
Of the potential patients in the study, 160 met the inclusion criteria, had no criteria for exclusion, and willingly agreed to participate. Most of the patients were females (60%), and the median age was 61.5 years (interquartile range [IQR]: 54-71). At the initial patients’ diagnosis, 90 (56.3%) were diagnosed with metaplasia and atrophy, 31 (19.4%) were diagnosed with atrophy, and 39 (24.3%) were exclusively diagnosed with metaplasia (Table 1).
Table 1 Characteristics of patients undergoing endoscopic check for premalignant lesions
| No injuries during check (n = 30) | Injuries during check (n = 130) | Total population (n = 160) | ||||
|---|---|---|---|---|---|---|
| Frequency | % | Frequency | % | Frequency | % | |
| Age (median/IQR) | 58.5 | (50-65) | 62 | (55-71) | 61.5 | (54-71) |
| Gender (female) | 21 | 70 | 75 | 57 | 96 | 60 |
| Initial diagnostic | ||||||
| Atrophy | 23 | 76.7 | 98 | 75.3 | 121 | 75.6 |
| Metaplasia | 23 | 76.7 | 106 | 81.5 | 129 | 80.6 |
| Endoscopic check diagnosis | ||||||
| H. pylori prevalence | 13 | 43.3 | 47 | 36.2 | 60 | 37.5 |
| Atrophy prevalence | 0 | 0 | 121 | 93.1 | 121 | 75.6 |
| Metaplasia prevalence | 0 | 0 | 113 | 86.9 | 113 | 70.6 |
| Premalignant lesions prevalence | 130 | 81.3 | ||||
| Medical history of PPI treatment | 1 | 3.3 | 17 | 13.1 | 18 | 11.3 |
| History of antibiotic treatment | 12 | 40 | 32 | 24.6 | 44 | 27.5 |
The histological result highlights that 81.3% of the patients showed some lesion degree (atrophy, metaplasia, dysplasia, or carcinoma), whereas 13 patients (18.7%) did not show any lesion in the biopsies performed. Similarly, a 37.5% prevalence of H. pylori was observed in the study population, with a higher prevalence in patients without lesions in the histological results (43.3%) than in patients with lesions (36.2%) (Table 1).
Furthermore, among the differences found between patients with and without lesions, there was a striking gender difference. There was a higher proportion of females among patients without lesions (70%) than among patients with lesions (57%). A much subtler disparity in age was also found, with a greater median age in patients with some lesions (62 years) compared to patients without lesions (58.5 years) (Table 1).
Of the patients with premalignant lesions, two patients had dysplasia. The first patient was a 72-year-old male. His initial diagnosis included metaplasia and atrophy. During the pathology result, he was diagnosed with atrophy, metaplasia, and high-grade dysplasia in the incisura, with no evidence of H. pylori in any of the biopsies. The second patient was a 61- year-old male. His initial diagnosis was metaplasia and atrophy, showing pathology consistent with high-grade dysplasia in the body, incisura, and antrum. He had atrophy and metaplasia in the antrum with signs of H. pylori in the body and incisura.
The histological results also showed a single 60-year-old female patient with carcinoma and an initial diagnosis of metaplasia. Her pathology resulted in carcinoma in the incisura, atrophy, and metaplasia in the antrum with H. pylori in the body.
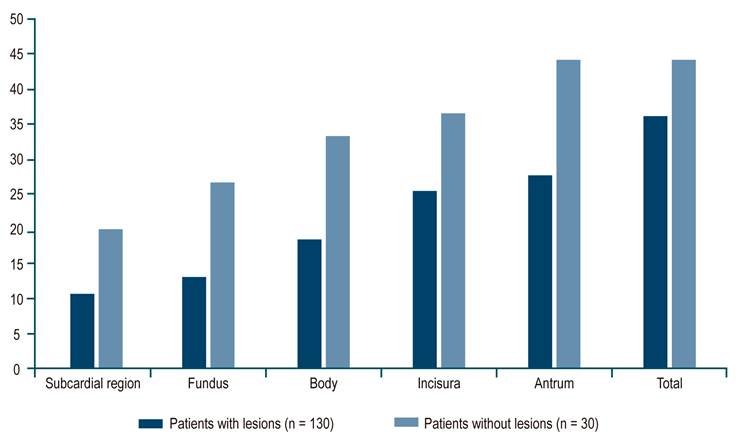
Based on the descriptive geographic analysis, it was possible to see that the prevalence of H. pylori increased from proximal to distal, starting with a 12.5% prevalence in the subcardial region and reaching a 30.6% prevalence in the antrum. A similar pattern was observed with the prevalence of preneoplastic lesions, starting with a low prevalence in the subcardial region (16.9%), decreasing at the fundus (11.9%), and with a progressive increase from that point to the antrum (66.2%) (Figure 1). In addition, there is a higher presence of advanced lesions (dysplasia, carcinoma) in the incisura (Table 2).
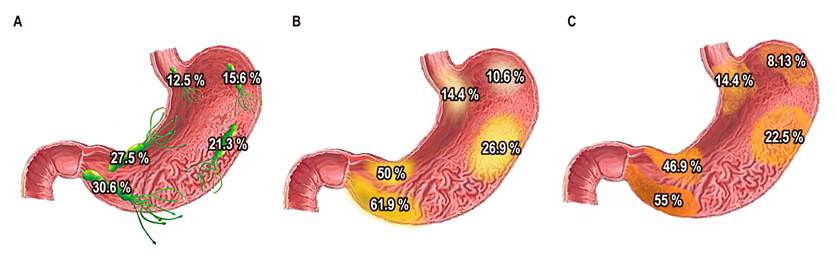
Figure 1 Prevalence and distribution of H. pylori (A), atrophy (B), and intestinal metaplasia (C) in patients with a previous diagnosis of preneoplastic conditions in Colombia.
Table 2 Diagnosis prevalence (%) by geographical location
| Diagnosis | Subcardial region | Fundus | Body | Incisura | Antrum | Total |
|---|---|---|---|---|---|---|
| H. pylori | 12.5 | 15.6 | 21.3 | 27.5 | 30.6 | 37.5 |
| Atrophy | 14.4 | 10.6 | 26.9 | 50 | 61.9 | 75.6 |
| Metaplasia | 14.4 | 8.13 | 22.5 | 46.9 | 55 | 70.6 |
| Dysplasia | 0 | 0 | 0.6 | 1.3 | 0.6 | 1.3 |
| Carcinoma | 0 | 0 | 0 | 0.6 | 0 | 0.6 |
| Neoplastic and pre-neoplastic lesions | 16.9 | 11.9 | 28.1 | 55.6 | 66.2 | 81.3 |
In the H. pylori intragastric distribution analysis with lesions, a higher prevalence of H. pylori was observed in all areas of the stomach in patients without lesions compared to patients with at least one lesion, showing a pattern of increasing prevalence from proximal to distal in both groups (Figure 2).
Discussion
The International Agency for Research on Cancer and the WHO classify H. pylori as a carcinogen type I10. Although the involvement of H. pylori in the carcinogenesis cascade of intestinal gastric cancer is accepted11, its prevalence in preneoplastic conditions such as intestinal atrophy and metaplasia, causing changes in the mucosal microenvironment -which could alter the gastric distribution of this pathogen, is currently unclear12. Currently, many authors recommend surveillance in patients with premalignant conditions through endoscopic monitoring and biopsies of the antrum, body, and incisura (Sydney protocol) every 2 to 3 years13. However, it is unclear whether or not H. pylori infection occurs in other gastric regions, which can lead to false negatives in pathogen identification -considering that several authors support the regression theory of these conditions8,14, pathogen identification would be critical to start an eradication treatment and, therefore, reduce the risk of gastric adenocarcinoma.
Approximately 50% of the world’s population is infected with H. pylori, ranging between 40% and 73%, with some variation depending on latitude9. In the 2016 study conducted by Dr. Correa in Medellín in a population with dyspeptic symptoms taken to endoscopy, a prevalence of up to 36.4% was found, with male predominance (39.6%) versus female (34%), with an average age of 46.515. There was a 36.2% prevalence of H. pylori in our population (patients with atrophy and metaplasia).
Although the global prevalence of H. pylori and its gastric distribution is known, its distribution in preneoplastic conditions such as atrophy and intestinal metaplasia is unclear. Therefore, the study was based on this pathogen’s location in the different gastric regions and found a higher increase of H. pylori in distal regions, which is more frequent in the antral region (30.6%) and lower in the subcardial region, with a 12.5% prevalence. When comparing the distribution of H. pylori in patients with atrophy and metaplasia versus patients in whom no premalignant entity was found, the pathogen observed in the latter group had a higher prevalence of the pathogen.
Regarding gastric atrophy prevalence, ranges reported in the literature vary between 9.4% and 63%. On the other hand, for intestinal metaplasia, the ranges reported were between 7.1% and 42.5%16-18. Park and Kim’s extensive review on premalignant entities in gastric cancer reported a 20.1% prevalence of gastric atrophy in the body and 42% in the antrum and a 21.2% prevalence of intestinal metaplasia in the body, and 28% in the antrum19. Our study found a similar distribution for atrophy and metaplasia in the gastric body (atrophy: 26%, intestinal metaplasia: 22%). However, a higher prevalence was observed in antral regions (atrophy: 61%, intestinal metaplasia: 55%). Notably, a lower prevalence of atrophy and metaplasia was also observed in proximal gastric areas, starting in the subcardial region (16.9%) and decreasing in the fundus (11.9 %) with a progressive increase up to the antrum (66.2 %).
Furthermore, the study conducted in Colombia in a high-risk population for gastric cancer reported a 39% intestinal metaplasia prevalence. Thus, showing a higher risk of gastric cancer in individuals with incomplete intestinal metaplasia and an extension of the metaplasia to the body and cardia. Although this study’s objective did not include evaluating the risk of adenocarcinoma according to the locations of the atrophy and metaplasia, two cases of patients diagnosed with dysplasia and one with adenocarcinoma were evidenced in the endoscopic check. They showed no H. pylori involvement due to atrophy or metaplasia in the gastric fundus or the subcardial region.
Our results suggest a low potential cost-benefit to performing control biopsies in the subcardial region and fundus, supporting the current suggested schemes. Nonetheless, this marginal benefit should be evaluated in longitudinal diagnostic studies to better assess the cost-effectiveness of biopsies in these two regions in patients with atrophy and metaplasia.
Among the strengths of this study is greater H. pylori diagnosis accuracy by covering all the anatomical areas of the stomach and a wide heterogeneity of the population as the study was conducted in a referral center in Colombia in a contributory regime population. Some limitations include the cross-sectional nature of the evaluation, so the times of patients with the disease are not standardized. Likewise, this study’s objective was to describe the H. pylori map in pre-malignant conditions, so making conclusions beyond the description of the population is not possible with the design.
Based on the evidence generated in this study, works of a longitudinal nature on a larger scale are recommended to measure H. pylori infection persistence or appearance impact in patients with preneoplastic entities more accurately. Moreover, studies should be conducted to evaluate the impact of biopsies in the fundus and subcardial region to establish control protocols in this population.
Conclusions
H. pylori prevalence in premalignant conditions was 36.2%, with a higher presence in distal than proximal regions. It is more frequent in the antral region and less in the subcardial region, which does not support the upward migration theory of H. pylori in these premalignant conditions.
As for the gastric distribution of atrophy and metaplasia, more involvement was found in the antrum and incisura and lower in the subcardial region and the fundus.
In Colombia, this is the first study that shows a complete mapping of the prevalence of atrophy, metaplasia, and H. pylori in a population with gastric premalignant entities.











 text in
text in