Introduction
Liver transplantation is a life-saving therapy in patients with end-stage liver disease. Multiple risk factors have been identified, and despite advances in immunosuppressive therapy and surgical techniques to improve post-liver transplant outcomes, graft rejection occurs between 23% and 64%1,2. It is imperative to understand the predictive factors related to adverse graft outcomes. Thus, identifying cardiovascular conditions without intervention before transplantation defines short- and long-term morbidity and mortality outcomes with the graft3,4. Post-transplant hemodynamic stress after reperfusion of the graft characterized by increased preload may result in multiple cardiovascular complications. The pre-transplant study protocol includes screening for traditional cardiovascular risk factors, coronary disease, and Doppler echocardiography analysis in the search for right or left ventricular dysfunction, portopulmonary hypertension, hepatopulmonary syndrome, and cirrhotic cardiomyopathy5,6.
Cirrhotic cardiomyopathy is an entity with no diagnostic criteria yet established. However, the best-accepted definition is that of the Cirrhotic Cardiomyopathy Consortium (2019), made up of variables such as systolic/diastolic alterations, supported by the assessment of global longitudinal shortening (GLS) and electrocardiographic changes such as QT prolongation7,8. This syndrome, which is usually not recognized in the initial phase, but rather in its decompensation, has gained importance in recent years as a predictor of outcomes such as heart failure, kidney injury, and even graft loss in the short and long term3,9-11. Data on cardiovascular complications and deaths from heart failure are found in up to 70% after transplantation12.
The discrepancy in some data, the insufficient evaluation of systolic/diastolic function parameters, incomplete data on the degree of diastolic dysfunction, imprecise determination of cardiac dysfunction in the final stage of cirrhosis with a physiological basis of a hyperdynamic state with high cardiac output, and non-adherence to echocardiographic assessment protocols limit the presentation of data in the literature9,11,13.
Due to the importance of hemodynamic assessment by echocardiography in its correlation with post-transplant outcomes such as heart failure, graft dysfunction, and mortality, for which no specific data can be found in the literature in our setting, we describe the experience of a leading Colombian hospital in liver transplantation.
Materials and methods
Study population and data collection
Retrospective cohort study. Data were obtained from the medical records of the liver transplantation group at the La Cardio hospital in Bogotá, Colombia, from January 1, 2005, to July 31, 2021.
Demographic data, paraclinical examinations, history, and conditions related to the surgical procedure were taken from each patient’s medical record.
Inclusion criteria
Patients older than 18 diagnosed with cirrhosis and stable disease undergoing liver transplantation.
Exclusion criteria
Patients with acute liver failure without cirrhosis requiring transplantation
Patients with retransplantation, transplantation of more than one organ, previous heart disease (ischemic or valvular)
Glomerular filtration rate (GFR) < 30 mL/min/1.73 (calculated using the Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI]).
Within the institutional pre-transplant assessment protocol, data were taken from Doppler echocardiograms based on the diastolic ventricular assessment protocol of the American Society of Echocardiography/European Association of Cardiovascular Imaging (ASE/EACVI) guidelines14. Patients who underwent right heart catheterization were analyzed.
Outcomes
Our primary outcome was early graft dysfunction, defined as an abnormal liver profile in the first seven days post-transplant, as follows: bilirubin >10 mg/dL, international normalized ratio (INR) > 1.5, and alanine aminotransferase (ALT) or aspartate aminotransferase (AST) > 2,000 IU.
Another primary outcome was acute kidney injury during post-transplant hospitalization based on the Kidney Disease: Improving Global Outcome (KDIGO) guideline definition.
Mortality was assessed from liver transplantation to the study completion date, July 31, 2021.
Other outcomes were considered, such as the requirement for renal replacement therapy during the post-transplant period, infectious complications, transfusion support, and intraoperative arrest.
Statistical analysis
The sociodemographic and clinical characteristics are presented in frequencies and percentages for the categorical variables. For continuous variables, we employed the mean with standard deviation (SD) when the distribution was normal or the median with interquartile range (IQR) when this criterion was not met. The chi-square test or Fisher’s test was performed to evaluate these comparisons according to the frequency of observations in the case of categorical variables. Student’s t-test for independent samples was used to compare continuous variables. A p-value < 0.05 was considered statistically significant.
We set up a classification and regression tree (CART) with all the variables collected for comparison purposes15. The covariates selection for the model was based on biological and clinical relevance, as previously reported in the literature, and their statistical significance in the bivariate analysis. The CART algorithm quantified the weight of each variable and built risk profiles. This methodology contrasts with classical regression models in which the CART algorithm can uncover modifier effects and complex interactions between variables. Statistical analysis was performed with R software version 3.6.3, and the CART model with the RPART (Recursive Partition and Regression Trees) package.
Results
Within the established date, the study found 550 patients with liver transplantation, of which 397 had complete data and met the inclusion and exclusion criteria.
General characteristics
Of the total number of patients, the median age was 56 years at the time of liver transplantation, and 54.4% were men. At the time of the transplant, 75% had a functional class I, and the most frequent history includes arterial hypertension (15.8%), diabetes mellitus (24.1%), and smoking (25.44%). A Charlson index with a mean of 4.4 (SD ± 1.5) was calculated.
According to the etiology of cirrhosis, the main one was alcoholic (17.8%), followed by cryptogenic (16%), hepatitis C virus (HCV) (15.3%), and autoimmune hepatitis (12.5%).
At the time of liver transplantation, complications due to the pathology produced at least one ascitic episode or more (63%), encephalopathy (47%), variceal bleeding (34.2%), hepatocellular carcinoma (22.1%), hepatopulmonary syndrome (15.6%) and spontaneous bacterial peritonitis (8.3%). Regarding the staging of the pathology, 52.3% were in Child-Pugh B and 26% in Child-Pugh C, with an average Model for End-Stage Liver Disease (MELD-Na) of 16 (SD ± 6) (Table 1).
Hemodynamic variables: echocardiography and right heart catheterization
Of the 397 patients analyzed, all were evaluated by 2D Doppler echocardiography. We found an average LVEF of 62% (SD ± 6.4), 71% with diastolic dysfunction, but only 45 patients had type 1 diastolic dysfunction, and seven had type 2 diastolic dysfunction; left ventricular hypertrophy (30.9%), and presence of shunt compatible with hepatopulmonary syndrome (19%). Right heart catheterization was only performed in seven patients (Table 1).
Table 1 Characteristics of the study population
| Patients, n | 397 |
| Age, years (median, IQR) | 56 (45-62) |
| Sex (male:female), n | 216:181 |
| BMI, kg/m2 (mean, SD) | 25.7 ± 4.2 |
| Functional class, n (%) | |
| - Class 1 | 298 (75) |
| - Class 2 | 90 (22.6) |
| - Class 3 | 8 (2) |
| - Class 4 | 1 (0.2) |
| Etiology of cirrhosis, n (%) | |
| - Alcoholic | 71 (17.8) |
| - Cryptogenic | 64 (16) |
| - HCV | 61 (15.3) |
| - Autoimmune hepatitis | 50 (12.5) |
| - NASH | 42 (10.5) |
| - Primary biliary cirrhosis | 39 (9.8) |
| - Secondary biliary cirrhosis | 13 (3.2) |
| - HBV | 13 (3.2) |
| - Other | 44 (11) |
| History, n (%) | |
| - Hypertension | 63 (15.8) |
| - Diabetes mellitus | 96 (24.1) |
| - COPD | 2 (0.5) |
| - Pulmonary hypertension | 3 (0.7) |
| - SLE | 4 (1) |
| - CKD | 30 (7.5) |
| - Smoking | 101 (25.44) |
| Complications of cirrhosis, n (%) | |
| - Ascites | 250 (63) |
| - Variceal bleeding | 136 (34.2) |
| - SBP | 33 (8.3) |
| - Hepatopulmonary syndrome | 62 (15.6) |
| - Encephalopathy | 187 (47) |
| - Pruritus | 19 (4.7) |
| - Hepatocellular carcinoma | 88 (22.1) |
| Child-Pugh score, n (%) | |
| - Class A | 86 (21.6) |
| - Class B | 208 (52.3) |
| - Class C | 103 (26) |
| - MELD-NA (mean, SD) | 16 ± 6 |
| Charlson index (mean, SD) | 4.4 ± 1.5 |
| Echocardiographic variables | |
| - LVEF (mean, SD) | 62 ± 6.4 |
| - TAPSE, n (mean, SD) | 22 (25 ± 4.3) |
| - DD, n (%) | 71 (17.8) |
| - Grade 1 DD, n (%) | 45 (11.33) |
| - Grade 2 DD, n (%) | 7 (1.7) |
| - Abnormal PASP, n (%) | 88 (22.2) |
| - Increased DBP, n (%) | 4 (1) |
| - LVH, n (%) | 130 (30.9) |
| - RVH, n (%) | 4 (1) |
| - Right ventricular dilatation, n (%) | 12 (3) |
| - Presence of shunt, n (%) | 79 (19) |
| Right catheterization variables (mean, SD) | n: 7 |
| - mPAP | 27.2 ± 9.5 |
| - PVR | 3.2 ± 3 |
| - Wedge pressure | 15.83 ± 5.3 |
| - Cardiac index | 4.3 ± 2.4 |
| - Right atrium pressure | 15.83 ± 5 |
| Intraoperative variables | |
| - Anhepatic phase (median, IQR) | 57 (47-69) |
| - Ischemic phase (median, IQR) | 6.3 (5.7-8.2) |
| - Intraoperative cardiac arrest, n (%) | 10(2.5) |
| - Days in ICU, median (IQR) | 2 (2-4) |
| - Transfusion requirement, n (%) | 244 (61.6) |
| Outcomes, n (%) | |
| Graft dysfunction, n (%) | 32 (8) |
| AKI, n (%) | 84 (21) |
| - KDIGO 1 AKI (%) | 37.9 |
| - KDIGO 2 AKI (%) | 32.1 |
| - KDIGO 3 AKI (%) | 29.88 |
| Dialysis requirement, n (%) | 29 (7.3) |
| Infection, n (%) | 116 (29.2) |
| - Abdominal | 49 (39.6) |
| - Pulmonary | 25 (21.5) |
| - Urinary | 21 (18.1) |
| Bacteremia | 6 (5.1) |
| Operative site | 5 (4.3) |
| Other | 10 (8.6) |
| Death, n (%) | 60 (15.1) |
DD: diastolic dysfunction; COPD: chronic obstructive pulmonary disease; CKD: chronic kidney disease; LVEF: left ventricular ejection fraction; RVH: right ventricular hypertrophy; LVH: left ventricular hypertrophy; BMI: body mass index; SLE: systemic lupus erythematosus; AKI: acute kidney injury; NASH: nonalcoholic steatohepatitis; DBP: diastolic blood pressure; SBP: spontaneous bacterial peritonitis; mPAP: mean pulmonary artery pressure; PASP: pulmonary artery systolic pressure; PVR: pulmonary vascular resistance; TAPSE: tricuspid annular plane systolic excursion; ICU: intensive care unit. Source: The authors.
Intraoperative and post-transplant variables
The anhepatic phase had a median of 57 minutes (IQR: 47-69), and the ischemic phase was 6.3 minutes (IQR: 5.7-8.2). Regarding the intraoperative complications, ten patients had a cardiorespiratory arrest, and 61.6% required transfusion support. The stay in the ICU was an average of two days. Only 32 patients presented with early graft dysfunction (8%); 21% presented acute kidney injury, and 29 required renal replacement therapy. Moreover, 29.2% exhibited infectious complications; the main ones were abdominal (39.6%) and pulmonary (21.5%). During the study period, there was a mortality of 15.1%.
Primary outcomes
The primary outcomes are summarized in Table 2.
Table 2 Univariate Cox analysis of primary outcomes
| Variables | Graft dysfunction | Kidney injury | Death | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No GD | GD | p | No | Yes | p | No | Yes | p | |
| Age (median, IQR) | 56 (45-62) | 52.5 (38.7-58.2) | 0.03405083 | 55 (44-61) | 58 (48.7-63) | 0.0626625 | 56 (45-62) | 58 (49-63) | 0.19050336 |
| Woman, n (%) | 158 (87.8) | 22 (21.1) | 0.01052545 | 151 (83.4) | 30(16.5) | 0.05438623 | 149 (82.3) | 32 (17.6) | 0.24357336 |
| Man, n (%) | 206 (95.3) | 10 (4.6) | 162 (75) | 54 (25) | 188 (87) | 28 (12.9) | |||
| BMI (mean, SD) | 25.8 ± 4.1 | 24.6 ± 4.9 | 0.11739833 | 25.5 ± 4 | 26.7 ± 4.6 | 0.04914905 | 26 ± 4.25 | 24.4 ± 3.9 | 0.01242746 |
| FC I | 276 (92.6) | 22 (7.3) | 0.55109023 | 234 (78.5) | 64 (21.4) | 0.24305443 | 251 (84.2) | 47 (15.7) | 0.09592338 |
| FC II | 80 (88.8) | 10 (11.1) | 72 (80) | 18 (20) | 79 (87.7) | 11 (12.2) | |||
| FC III | 8 (100) | 0 | 7 (87.5) | 1 (12.5) | 7 (87.5) | 1 (12.5) | |||
| FC IV | 1 (100) | 0 | 0 | 1 | 0 | 1 | |||
| Charlson index (mean, SD) | 4.47 ± 1.55 | 4.06 ± 1.1 | 0.16534394 | 4.32 ± 1.5 | 4.7 ± 1.5 | 0.0372914 | 4.3 ± 1.5 | 4.7 ± 1.6 | 0.15445272 |
| Etiology of cirrhosis, n (%) | |||||||||
| Alcoholic | 69 (97.1) | 2 (2.8) | 0.13026919 | 55 (77.4) | 16 (22.5) | 0.9251709 | 63 (88.7) | 8 (11.2) | 0.67038578 |
| Cryptogenic | 60 (93.7) | 4 (6.2) | 53 (82.8) | 11 (17.1) | 56 (87.5) | 8 (12.5) | |||
| HCV | 57 (93.4) | 4 (6.5) | 44 (72.1) | 17 (27.8) | 47 (77) | 14 (22.9) | |||
| Autoimmune hepatitis | 47 (94) | 3 (6) | 40 (80) | 10 (20) | 43 (86) | 7 (14) | |||
| NASH | 39 (92.8) | 3 (7.1) | 31 (73.8) | 11 (26.1) | 37 (88) | 5 (12) | |||
| PBC | 33 (84.6) | 6 (15.3) | 33 (84.6) | 6 (15.3) | 31 (79.4) | 8 (20.5) | |||
| SBC | 11 (84.6) | 2 (15.3) | 11 (84.6) | 2 (15.3) | 9 (69.2) | 4 (30.7) | |||
| HBV | 12 (92.3) | 1 (7.6) | 10 (76.9) | 3 (23) | 12 (92.3) | 1 (7.6) | |||
| History, n (%) | |||||||||
| Hypertension | 60 (95.2) | 3 (4.7) | 0.42588466 | 48 (76.1) | 15 (23.8) | 0.69396299 | 56 (88.8) | 7 (11.1) | 0.43822945 |
| Diabetes mellitus | 91 (94.7) | 5 (5.2) | 0.33522914 | 72 (75) | 24 (25) | 0.360296 | 82 (85.4) | 14 (14.5) | 0.99769806 |
| COPD | 2 (100) | 0 | 1 | 1 | 1 | 0.89392224 | 2 | 0 | 1 |
| PH | 3 (100) | 0 | 1 | 3 | 0 | 0.8483549 | 3 | 0 | 1 |
| SLE | 4 (100) | 0 | 1 | 2 | 2 | 0.4212489 | 3 | 1 | 1 |
| CKD | 14 (100) | 0 | 1 | 10 (71.4) | 4 (28.5) | 0.72013665 | 12 (85.7) | 2 (14.2) | 1 |
| Smoking | 97 (96) | 4 (3.9) | 0.12324534 | 78 (77.2) | 23 (22.7) | 0.74992213 | 88 (87.1) | 13 (12.8) | 0.57024989 |
| Complications of cirrhosis, n (%) | |||||||||
| Ascites | 233 (93.2) | 17 (6.8) | 0.3114414 | 194 (77.6) | 56 (22.4) | 0.507671 | 215 (86) | 35 (14) | 0.50759422 |
| Variceal bleeding | 124 (91.1) | 12 (8.8) | 0.83450994 | 110 (80.8) | 26 (19.2) | 0.55567329 | 120 (88.2) | 16 (11.7) | 0.23129388 |
| SBP | 31 (93.9) | 2 (6) | 0.91493425 | 25 (75.7) | 8 (24.2) | 0.81777885 | 30 (90.9) | 3 (9) | 0.45028031 |
| Hepatopulmonary syndrome | 58 (93.5) | 4 (6.4) | 0.80053717 | 54 (87) | 8 (12.9) | 0.11797885 | 56 (90.3) | 6 (9.6) | 0.26790479 |
| Encephalopathy | 169 (90.3) | 18 (9.6) | 0.3700466 | 139 (74.3) | 48 (25.3) | 0.05082367 | 160 (85.5) | 27 (14.4) | 0.83062933 |
| Pruritus | 16 (84.2) | 3 (15) | 0.40289368 | 19 | 0 | 1 | 16 (84.2) | 3 (15.7) | 1 |
| Hepatocellular carcinoma | 84 (95.4) | 4 (4.5) | 0.24972757 | 67 (76.1) | 21 (23.8) | 0.57802013 | 68 (77.2) | 20 (22.7) | 0.03647202 |
| Child-Pugh score, n (%) | |||||||||
| Class A | 79 (91.8) | 7 (8.1) | 0.48422153 | 65 (75.5) | 21 (24.4) | 0.66648793 | 71 (82.5) | 15 (17.4) | 0.64598336 |
| Class B | 194 (93.2) | 14 (6.7) | 167 (80.2) | 41 (19.7) | 176 (84.6) | 32 (15.3) | |||
| Class C | 92 (89.3) | 11 (10.6) | 81 (78.6) | 22 (21.3) | 90 (87.3) | 13 (12.6) | |||
| MELD-NA (mean, SD) | 15.9 ± 6.8 | 17.4± 7.7 | 0.28508139 | 15.8 ± 6.4 | 16.6 ± 8.5 | 0.89719791 | 16 ± 7 | 15.9 ± 8.2 | 0.50753706 |
| Echocardiographic variables | |||||||||
| LVEF (mean, SD) | 62.39 ± 6.5 | 61.2 ± 5.4 | 0.92794776 | 61.7 ± 6.5 | 64.4 ± 5.8 | 0.00059796 | 62 ± 6.3 | 64.1 ± 6.9 | 0.03786134 |
| TAPSE, n (mean, SD) | 25.5 (4.4) | 25.3 | 0.93718292 | 25.3 | 30.1 | 0.18031438 | |||
| Diastolic dysfunction, n (%) | 65 (91.4) | 6 (8.4) | 1 | 49 (69) | 22 (30.9) | 0.03780355 | 57 (80.2) | 14 (19.7) | 0.3112245 |
| PASP | 80 (90.9) | 8 (9) | 0.86944739 | 68 (77.2) | 20 (22.7) | 0.81631308 | 68 (77.2) | 20 (22.7) | 0.03881794 |
| Increased DBP, n (%) | 3 (75) | 1 (25) | 0.74304706 | 4 | 0 | 0.67000042 | 3 | 1 | 1 |
| LVH, n (%) | 110 (89.4) | 13 (10.5) | 0.30260062 | 83 (67.4) | 40 (32.5) | 0.00034267 | 98 (79.6) | 25 (20.3) | 0.07329209 |
| RVH, n (%) | 4 (100) | 0 | 1 | 3 | 1 | 1 | 3 | 1 | 1 |
| RV dilation, n (%) | 12 (100) | 0 | 0.60608871 | 10 (83.3) | 2 (16.6) | 0.97293598 | 12 | 0 | 0.2751521 |
| Presence of shunt, n (%) | 71 (89.8) | 8 (10.1) | 0.60107922 | 60 (75.9) | 19 (24.05) | 0.58281122 | 66 (83.5) | 13 (16.4) | 0.84406117 |
| Intraoperative variables | |||||||||
| Anhepatic phase (median, IQR) | 57 (47-68) | 60 (50-77) | 0.16471272 | 56 (46-66) | 60 (51 -75) | 0.01654853 | 57 (47-68) | 56 (50-73) | 0.60601576 |
| Ischemic phase (median, IQR) | 6.3 (5.1-8.1) | 6.4 (5.3-9) | 0.54891242 | 6.3 (5.2-8.3) | 6.1 (5-7.5) | 0.3038509 | 6.2 (5-8) | 6.9 (5.4-9) | 0.05746276 |
| Transfusion requirement, n (%) | 219 (89.7) | 25 (10.2) | 0.03781268 | 189 (77) | 55 (26) | 0.48819885 | 205 (84) | 39 (15.9) | 0.53335917 |
| AKI, n (%) | 71 (84.5) | 13 (15.4) | 0.00970643 | 12 (41.3) 17 (58.6) | 56 (66.6) | 28 (33.3) | 3.79E-07 | ||
| Dialysis requirement, n (%) | 21 (72.4) | 8 (27.5) | 0.00027098 | 7.9935E-11 | |||||
| Infection, n (%) | 104 (89.6) | 12 (10.3) | 0.38345075 | 78 (67.2) | 38 (32.7) | 0.00046397 | 87 (75) | 29 (25) | 0.00072601 |
PBC: primary biliary cholangitis; SBC: secondary biliary cholangitis; FC: functional class; GD: graft dysfunction; PH: pulmonary hypertension; HBV: hepatitis B virus. Source: The authors.
Early graft dysfunction
Within the univariate Cox analysis, a relationship was found with the female sex (p = 0.010), transfusion support requirement (p = 0.037), acute kidney injury (p = 0.0097), and renal replacement therapy requirement (p = 0.0002).
Acute kidney injury
It was related to the male sex (p = 0.054), BMI (p = 0.049), Charlson index (p = 0.0372), episodes of encephalopathy before transplantation (p = 0.0508), LVEF (p = 0.00059), diastolic dysfunction (p = 0.037), LVH (p = 0.00034), anhepatic phase (p = 0.016), and infection (p = 0.0004).
Mortality
It was associated with hepatocellular carcinoma (p = 0.036), acute kidney injury (p < 0.005), renal replacement therapy (p < 0.005), and infection (p < 0.005).
CART predictive model
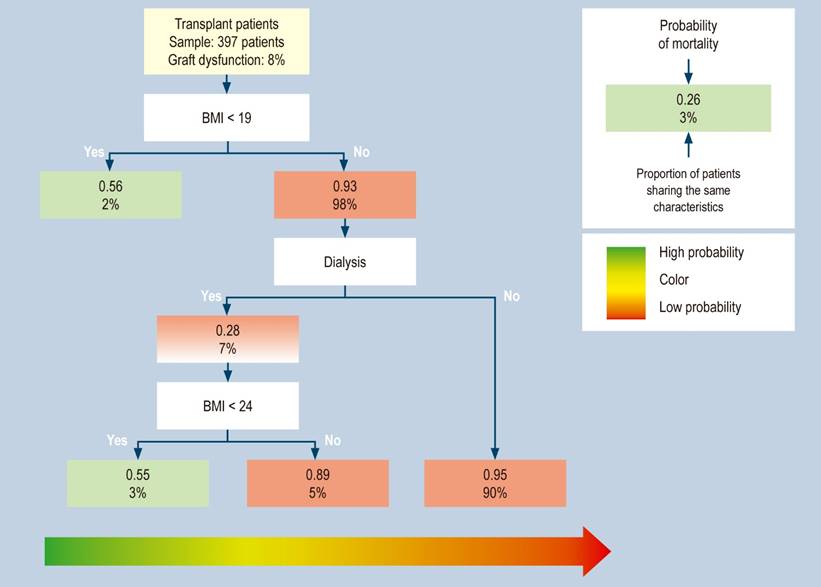
This method built a predictive model with mortality during the study, graft dysfunction, and acute kidney injury variables. The mortality during the study model found a mortality of 15%, in which patients with BMI < 19 had a 56% probability of dying. Meanwhile, with a BMI > 19 (98% of patients with fatal outcomes), patients who do not require dialysis have a 95% chance of survival. However, patients with a BMI > 19 and < 24 and requiring dialysis have a 55% chance of dying (Figure 1).
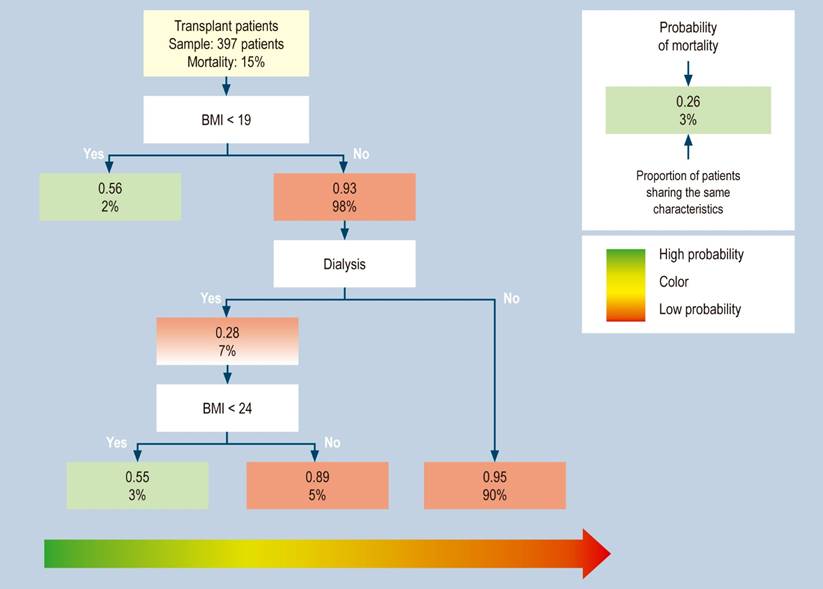
Figure 1 Distribution of transplant patients with mortality during the study, classified by risk groups in the regression tree (CART). This method builds a predictive model of three risk profiles with BMI < 19 and < 24, with or without dialysis requirement. Source: The authors.
Concerning graft dysfunction, they were 8% of all patients. Patients with BMI < 19 had a 56% chance of graft dysfunction, and those with BMI > 19 and < 24 requiring dialysis had a 55% chance of death (Figure 2).
Discussion
Globally, in 2017, approximately 1.5 million people had liver cirrhosis, whose main etiologies were NASH (60%), HBV (29%), HCV (9%), and alcoholic cirrhosis (2%); it produced 1.2 million deaths and was 3.5% of all causes of death16. In data from local studies, it gains importance as an etiology of alcoholic cirrhosis, as shown in our study17. There are multiple risk factors in the pre-transplant study, and even its poor selection is related to a cost burden for the healthcare system18. Pretransplant detection of cardiac dysfunction is a predictor of adverse events after liver transplantation, based on analysis of systolic or diastolic abnormalities9,11,19. Cardiac dysfunction leads to early mortality from cardiovascular causes (40%), followed by other causes of mortality, such as infections (27.2%) and graft rejection (12%)20. Even data have shown liver transplantation as a treatment for cardiovascular disorders, as reported by a study in which a decrease in biventricular dilatation and improvement in global strain post-transplantation were observed21.
Regarding the findings in our study, no relationship was identified between LVEF and mortality during follow-up. The data are similar to the literature, in which no relationship was found with the mortality or post-transplant cardiac arrest outcomes9. However, in another study, a low LVEF is related to mortality22; even in the hyperdynamic state, it correlates with high LVEF, whose minimal variations are related to cardiovascular outcomes10,22. Therefore, in assessing a patient with a hyperdynamic state associated with decreased peripheral vascular resistance, not only the evaluation of the systolic component becomes vital for diagnosing cirrhotic cardiomyopathy. An integration of variables such as those stipulated in the last CCC classification, with findings of LVEF < 50% and decrease in GLS > 18%, is also relevant, with careful assessment of the diastolic component considering the multiple variables that can affect preload based on electromechanical abnormalities such as QT prolongation8.
Due to the complex assessment of cardiac dysfunction, there is no protocolized evaluation of this specific condition by echocardiography without following the latest recommendations for diagnosing cirrhotic cardiomyopathy. According to our study, there is no detail of the diastolic evaluation without the GLS and electromechanical component measurements. In the literature, systolic involvement is low, as shown in our study, where 100% of patients did not meet the criteria for systolic changes22. Some studies even reveal an incidence of only 2% of systolic involvement13.
Although the CCC’s latest classification of cirrhotic cardiomyopathy has an LVEF cut-off < 55%, data of LVEF < 60% in patients undergoing immunosuppression should have a closer follow-up, as it is a predictor of mortality and cardiovascular outcomes23.
Among the findings of our study is that 17.8% presented with diastolic dysfunction, which does not agree with the literature since it shows a varied prevalence, possibly secondary to the non-standardization of echocardiographic variables. Reports in the literature have demonstrated that up to 66% of patients with end-stage liver disease have, according to the ASE classification, type 1 (53%) and type 2 (47%) diastolic dysfunction, with no type 3 patients22, as found in our study (no findings of type 3 patients). Another study shows a prevalence similar to ours with data of diastolic dysfunction of 19%: mild (48%), moderate (30%), and severe (22%), and the findings of pre-transplant diastolic dysfunction were related to the risk of graft rejection, graft failure, and mortality3. Nevertheless, in our study, the finding of diastolic dysfunction was related to the development of acute kidney injury (p = 0.0378).
Additional echocardiographic variables, such as left atrial volume index (LAVI) > 40 mL/m2, were associated with the risk of mortality within one year post-transplant9. The rate of moderate to severe tricuspid regurgitation is related to mortality since mild findings are expected in the patient’s hyperdynamic state24; our study’s data were not measured because they were found to be normal. Other findings are LVH, which occurs in 12% to 30% of patients with cirrhosis, an indication of possible diastolic dysfunction in the context of left ventricular remodeling in the patient’s hyperdynamic state; in our study, it was found in 30.9% of patients, with no relationship with mortality outcomes or graft dysfunction. These data differ from those in the literature, in which LVH was associated with mortality nine months after transplantation; it was more frequent in the elderly and patients with a history of arterial hypertension25. Even its presence before transplantation has been observed as a predictor of post-transplant echocardiographic deterioration26. However, the relationship between LVH and diastolic dysfunction was related to acute kidney injury, as shown by studies related to a low cardiac index in severe arterial vasodilation changes22,27,28; some data even show a correlation between a high LVEF and the deteriorating renal function possibly secondary to this hyperdynamic state29. Progressing to the requirement of renal replacement therapy was related in our study to graft dysfunction and mortality, as found in the literature, in which its relationship with mortality was noted, with an odds ratio (OR) of 14.18 (confidence interval [CI] 1.65-121.89; p < 0.05)11.
Within our study, increased PASP was observed in 22% of the patients, which was related to mortality with p = 0.038; this finding may be related to increased left ventricular diastolic pressure and, therefore, be a marker of diastolic dysfunction. In one study, it was connected to the risk of cardiac events (hazard ratio [HR]: 1.79 [1.48-2.17]; p < 0.001)29 and has even been directly associated in some studies with pulmonary artery pressure with catheterization, allowing for adequate screening with a detailed echocardiogram30.
The transfusion requirement in the post-transplant period was related to graft dysfunction, and in the regression tree, to acute kidney injury in the initial stages and progression to dialysis. Previous studies have associated these findings with adverse post-transplant outcomes31. Another variable related to mortality and acute kidney injury was a post-transplant infection. These data are related to the literature concerning the immunosuppression state due to the pathology and the level of immunological activation to the infectious stimulus in a patient with a chronic inflammatory disease and hemodynamic dysregulation, causing mortality rates close to 50%32.
Within the CART, the critical data related to mortality and graft dysfunction are low BMI, with a cut-off < 19 in our study, even related to renal replacement therapy. These data indirectly indicate sarcopenia since they show that 30%-70% of individuals with cirrhosis suffer from this condition due to the patient’s degree of inflammation, chronic bacterial translocation, insulin resistance, hyperammonemia, and decreased testosterone. Previous data condition higher mortality (19 ± 6 months with sarcopenia vs. 34 ± 11 months without sarcopenia; p = 0.005)33,34. Even data already related to post-transplant outcomes of skeletal muscle indices measured by tomography were linked to lower post-transplant survival35.
Conclusions
Pre-transplant variables, from echocardiogram aids to previously associated conditions such as sarcopenia, considerations during liver transplantation, and the requirement or not of renal replacement therapy related to acute kidney injury are points of intervention and follow-up to reduce long-term complications and even impact the mortality of these patients.











 text in
text in