Introduction
Chronic hepatitis C is a significant public health challenge. In 2019 approximately 290,000 people died of hepatitis C, mainly due to the development of end-stage liver disease (cirrhosis and hepatocellular carcinoma). There are currently 58 million people infected with the hepatitis C virus (HCV), and around 1.5 million new infections are reported yearly1.
Chronic HCV infection triggers an inflammatory process that can progress to liver fibrosis, cirrhosis, hepatocellular carcinoma, and death2. Cirrhosis and liver decompensation are associated with a 2%-5% annual risk of death, and 15%-20% of people with liver disease die within the first year after decompensation3. In addition, chronic hepatitis C is one of the West’s leading causes of liver transplantation (Figure 1)4-6.
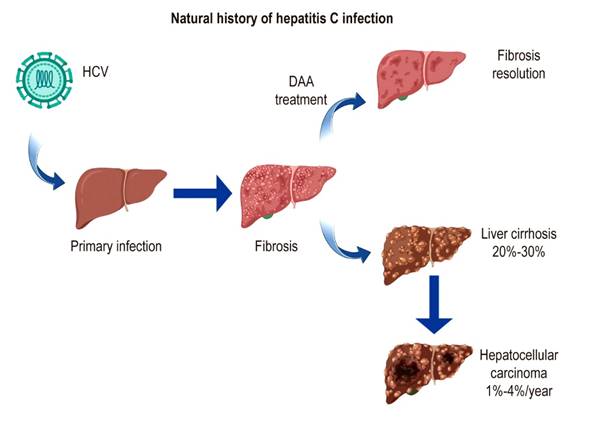
Figure 1 Natural history of hepatitis C infection. DAAs: direct-acting antivirals. Adapted from Lingala et al3.
The World Health Organization (WHO) has established a global strategy against viral hepatitis. Its objectives include diagnosing 90% of cases and treating 80% of patients, achieving a 65% reduction in mortality associated with viral hepatitis by 20307. Actively searching for cases with good admission to the healthcare system is imperative to accomplish these objectives since this information can guide interventions focused on reducing this disease in risk groups8.
In addition to an active search and an adequate characterization of the population, it is necessary to have an antiviral treatment regimen to meet the WHO goal of control and elimination. An indication of cure of HCV infection is when a sustained viral response (SVR) is demonstrated, which is defined as the absence of the HCV genome in serum or plasma by assay (LLOD ≤ 15 IU/mL) at week 12 (SVR12) or week 24 (SVR24) after finishing the treatment. Viral clearance is also associated with normalizing serum levels of liver enzymes, improvement or disappearance of hepatic necroinflammation and fibrosis, and a decrease in extrahepatic manifestations of HCV infection9.
Highly effective drugs are required to achieve this SVR. For a long time, the only therapy available for patients with hepatitis C was based on type I interferon. It had many limitations due to side effects and viral resistance, leading to therapy failure in many patients10. However, there are currently direct-acting antivirals (DAAs) that target viral proteins such as NS3 and its cofactor NS4A (protease inhibitors), NS5B (nucleoside or non-nucleoside viral polymerase inhibitors), and NS5A (viral replication complex inhibitors)11. These antivirals have a viral clearance rate close to 95%, even in cases of human immunodeficiency virus (HIV) coinfection, decompensated liver cirrhosis, and end-stage renal disease12,13. DAA treatment regimens can be aimed at a specific genotype or pan-genome, which is the current preference. Viral genome sequencing (NS3, NS5A, and NS5B) before treatment is recommended in some cases where the risk of resistance is high for specific DAA regimes10.
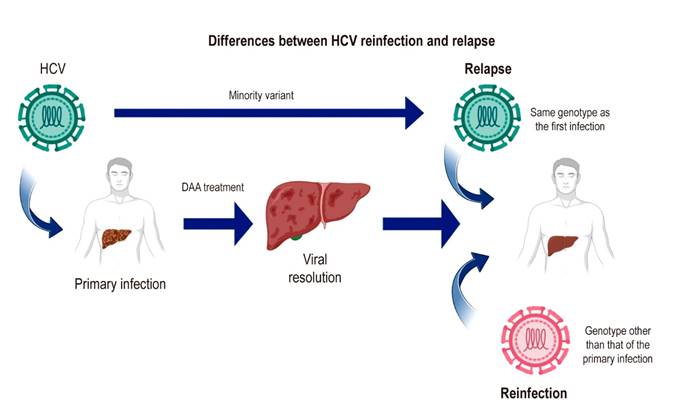
A recurrence (relapse or reinfection) should be suspected in case of a reappearance of viremia after SVR14. If it occurs after SVR12, it may be due to relapse, in which HCV persists in the individual and reappears due to the selection of a minority viral variant. This variant results from HCV RNA-dependent viral RNA polymerase activity that has no proofreading activity and therefore has a high error rate, resulting in genetic heterogeneity and the creation of quasispecies (Figure 2)15. In addition, late relapse has been described in some cases, detailing undetectable viral load levels after treatment (SVR) and the reappearance of the viral genome in the blood 24 weeks after the end of the DAA treatment regimen. Late relapse cases have been documented using deep sequencing; this methodology calculates genetic diversity in terms of the number of mutations per site in the quasispecies of the HCV genome isolated from a patient before treatment and during late relapse. This analysis is needed to prove a late relapse or reinfection with the same HCV genotype as the first infection because of the implications of the treatment regimen and creates the need to reconsider the follow-up time of a patient after completing the treatment regimen16,17. Late relapse is a phenomenon facilitated by HCV infection of extrahepatic sites, as documented in peripheral blood mononuclear cells, dendritic cells18,19, and cells of the gastrointestinal mucosa20.
The possibility of reinfection should be considered in an individual after completing the treatment regimen with viral clearance. One of the most critical factors associated with reinfection is a humoral and cellular immune response with little activity against HCV. It should be noted that the response is specific to the viral genotype and, therefore, is susceptible to HCV genotypes other than that of the first infection21-23. The viral genotype should be characterized before and after treatment to distinguish between relapse and reinfection. Since reinfection corresponds to a new infection by another HCV genotype, the diagnosis of reinfection is established by detecting an HCV genotype different from the first infection24-26.
With DAAs, virologic relapse rates after SVR12 are meager. Cases of a virologic relapse generally occur within the first four weeks after the treatment regimen, while reinfection can be defined as the detection of HCV viremia in persons with an SVR12 after completing treatment with a DAA regimen who have risk factors8.
According to the European Association for the Study of the Liver (EASL), patients who achieve SVR should undergo viral load monitoring 24 weeks after completing treatment, mainly in populations with risk factors, such as people who inject drugs (PWID) and men who have sex with men (MSM). The cure for the infection can be deemed definitive when the HCV genome is undetectable at the time27.
The American Association for the Study of Liver Diseases (AASLD) recommends check-ups every 6-12 months in people with risk factors or unexplained liver dysfunction to assess for HCV relapse or reinfection28.
This article presents the first case of HCV reinfection in a patient with an SVR after DAA treatment.
Hepatitis C virus
HCV was discovered in 1989, and since then, clinical and basic science research has enabled progress in understanding its pathogenesis and the development of tools for diagnosis and treatment29,30. So far, eight HCV genotypes and more than 90 subgenotypes have been characterized31,32. HCV is a member of the family Flaviviridae, genus Hepacivirus. The viral genome is a single-stranded, positive-sense RNA of approximately 9.6 kb, encoding a single polyprotein cleaved into ten proteins: three structural proteins, the capsid protein (core), and two glycoproteins present in the envelopes (E1 and E2), as well as seven non-structural proteins, including two proteins necessary for virion assembly (p7 and NS2) and five proteins that form the viral replication complex (NS3, NS4A, NS4B, NS5A, and NS5B)10.
Hepatitis C infection transmission and risk factors
HCV is primarily transmitted parenterally. It generally requires exposure to blood through transfusions, an iatrogenic route, the reuse of needles and syringes, and vertical transmission33,34.
The sexual transmission of HCV has been documented and associated with factors such as unprotected sex, fisting, and group sex. Furthermore, biological risk factors have been described in the literature, such as ulceration or concurrent sexually transmitted infections and human immunodeficiency virus (HIV) infection35,36. An increase in HCV cases acquired by this route has been observed since the 2000s, particularly in men who have sex with men (MSM) and people with HIV infection37.
The importance of knowing the associated risk factors has been demonstrated in the study by Knick et al., in which they identified that the regions with a high incidence of HCV had less information about the risk factors than the inhabitants of low-incidence regions38.
Natural history of hepatitis C virus
Hepatitis C is characterized by a transient infection in 15%-40% of cases and chronic infection in 60%-85% of cases. Spontaneous clearance of the infection involves an early, functional, sustained cellular and humoral immune response directed against multiple epitopes of viral proteins and is associated with host and virus variables21-23.
Most patients develop specific antibodies against HCV in the early phase of infection. However, the role of neutralizing antibodies directed against conformational epitopes located on E1 and E2 glycoproteins in viral clearance is still controversial23.
One of the evasion strategies of the immune response by the virus is the high genetic variability of HCV represented in the number of quasispecies throughout the evolution of the infection in an individual. The mechanisms described in persistent HCV infection include the depletion phenotype of CD8+ T cells (CD127low PD-1high), decreased activity of CD4 T cells to express inhibitory molecules, such as programmed cell death protein 1 (PD-1), T cell immunoreceptor with Ig and immunoreceptor tyrosine-based inhibition motif domains (TIGIT), and cytotoxic T lymphocyte antigen 4 (CTLA-4), and the production of immunomodulatory cytokines, such as IL-10 and TGF-β by Treg lymphocytes21-23.
After DAA treatment, an increase in the frequency of HCV-specific CD8+ T cells and a partially restored proliferative capacity have been described. These changes are due to the maintenance of a memory-like CD8+ T cell population and the disappearance of CD8+ T cells with a terminal depletion profile after HCV clearance. However, memory-like cells are not fully restored to the level of conventional memory-like CD8+ T cells, which sets up a pattern of functional exhaustion21-23.
Reinfection risk factors
A meta-analysis of 61 studies estimated reinfection rates of 0.95%, 10.67%, and 15.02% at five years in the low-risk, high-risk, and HIV coinfection groups, respectively34. Notably, most studies included in this review evaluated recurrence after treatment with interferon-based therapies.
Few studies assess reinfection rates in the DAA era; however, some experts speculate that DAA treatment may be associated with higher rates of reinfection due to the availability of antivirals, fewer adverse effects, and a high probability of cure, which may encourage risky behavior in people after healing from a first infection8. Ingilis P et al. published a retrospective cohort study that evaluated MSM reinfection in patients treated with DAAs; the authors found that the risk of reinfection in the study population was higher than in the control group (1.89 per 100 person-years; 95% confidence interval [CI]: 1.41-2.48) and that the reinfection rate was higher in this population treated with DAAs than in patients treated with interferon, although without statistical significance (hazard ratio [HR]: 1.05; 95% CI: 0.64-1.74; p = 0.831)39.
For its part, a cohort study by the Madrid Registry (Madrid-CoRe Study) on patients with HIV/HCV coinfection treated with DAAs detected 12/177 cases of MSM reinfection (6.8%), representing an incidence rate of 5.93 per 100 people/year (95% CI: 3.37-10.44). In the same cohort, there were 5/1,459 cases of reinfection in PID (0.21 per 100 people/year)40. In a similar study, Smit and colleagues described the findings of the ATHENEA study from 2000 to 2019. ATHENEA is a national HIV infection observation registry established in 1998 that collects clinical data from adults and children in the Netherlands. Of a total of 23,590 cases, 1,269 diagnoses of primary HCV infection (incidence: 5.2 per 1,000 person-years [95% CI: 5.0-5.5]) and 274 HCV reinfections (incidence: 26.9 per 1,000 person-years [95% CI: 23.9-30.3]) were documented41,42.
It has been described that factors such as heavy alcohol use, reuse of needles and syringes, anal sex, and group sex are associated with HCV reinfection in patients with HIV infection and MSM. Moreover, it has been shown that mental health counseling reduces the risk of HCV reinfection (aHR: 0.24; 95% CI: 0.13-0.46)43.
Prevention of hepatitis C reinfection
One condition that facilitates reinfection is the absence of promotion and prevention strategies; consequently, this problem represents a significant burden for the health system and patients. Considering that no vaccine is available, the cornerstone of HCV infection control and elimination is DAA treatment, and therefore more pragmatic strategies have been proposed to avoid reinfection. Methods such as micro-elimination consist of adapting the WHO elimination objectives to small groups to address segments of the population individually and specifically. Thus, treatment and prevention interventions could be made more quickly and efficiently and achieve HCV micro-elimination in specific subpopulations, such as people with HIV coinfection or inmates44. An example of this strategy is the Swiss HCVree trial, which aimed to evaluate this strategy in patients with HCV/HIV coinfection and MSM. The high rate of persistence of HCV infection, in combination with the high risk of transmission in these groups, make antiviral treatment a preventive intervention and a way to achieve HCV micro-elimination in MSM. This micro-elimination program resulted in a 57% and 84% decrease in the incident and prevalent HCV infections, respectively45, over two years.
Adequate follow-up of patients after DAA treatment is also found in reinfection prevention strategies, as shown in a study in Japan. The follow-up of 1,392 patients with SVR was conducted via viral load in a sample obtained every six months. Of the total number of patients in follow-up, 434 (31.2%) continued with regular visits for more than ten years; none of the follow-up patients were positive during the study, demonstrating the importance of strict clinical surveillance46.
The ideal prevention strategy for HCV infection is vaccination; however, the different evasion mechanisms of the HCV immune response, including genetic variability, have been a significant impediment to developing a vaccine21-23.
Meanwhile, coinfection with two or more HCV genotypes has been described, particularly in populations such as PID, MSM, and individuals with HIV coinfection, among others. Of note is that the methods used in clinical practice limit the possibility of adequately demonstrating coinfections with two or more HCV genotypes, mainly when Sanger-type sequencing strategies are used, which generally only identify the predominant genotype. On the contrary, new-generation sequencing technologies allow the reading of all sequences, differentiating the majority genotype from the minority one.
In any case, pan-genotypic antiviral therapy is recommended in the vast majority of cases of reinfection or coinfection due to its high probability of SVR, which is a great advantage in developing countries, where the sequencing of a new generation is not very accessible. Nonetheless, the possibility of therapeutic failure should always be considered in case of coinfection with more than one genotype47.
Another pillar in the multidisciplinary management of hepatitis C is the adequate screening and assessment of the patient’s sexual partners to offer antiviral treatment and reduce incidence and complication rates. Although the patient is responsible for contacting and informing their partners, liaising with the health sector is necessary to offer education and opportunities to prevent and treat the disease.
The incidence rate of HCV infection in seronegative partners of individuals with a chronic condition has been described as 0%-0.6% in prospective cohort studies of monogamous heterosexual couples. Therefore, a higher incidence is expected in individuals with multiple partners; however, the study results are probably controversial because sometimes, recording risk factors and information about sexual partners is complex 48,49.
Hepatitis C reinfection treatment
The effectiveness of DAA therapy for HCV infection is supported by both randomized controlled trials and real-life studies; still, in terms of HCV reinfection, the available data on the effectiveness of treatment is minimal, and as described above, reinfection can compromise the benefits of cure at the individual and population levels if not adequately treated50.
The EASL recommendation on reinfection is to offer a new course of DAA treatment, with a three-month waiting period to allow possible spontaneous elimination, except if urgent treatment is needed14.
The REACH-C study is an observational study that evaluated DAA treatment outcomes in 33 health services in Australia between March 2016 and June 2019. Of the 10,843 individuals starting DAA for the first time, 99 reinfections were reported. Treatment for reinfection occurred in 88 individuals for whom no resistance studies were requested. Regimens used to treat reinfection included glecaprevir/pibrentasvir (50%), sofosbuvir/velpatasvir (36%), sofosbuvir/ledipasvir (5%), sofosbuvir/daclatasvir (5%), grazoprevir/elbasvir (3%), and sofosbuvir/velpatasvir/voxilaprevir (1%).
The primary outcome was SVR12, similar to treatment for primary infection (95% vs. 95%; p = 0.745) and comparable in settings of primary, tertiary, and prison levels of complexity. The study concludes that the treatment of reinfection with pan-genotypic DAAs is highly effective and can be administered in non-specialized settings50.
Still, Colombia only provides the sofosbuvir/velpatasvir combination (pan-genotypic regimen) as a therapy for treating HCV infection through the high-cost account due to its high effectiveness, the possibility of use even in patients with severe renal dysfunction, and the centralized purchase of drugs. Besides, because of the characteristic of the pan-genotypic regimen in Colombia, performing a genotype test before starting treatment is not recommended by clinical practice guidelines51.
The preceding implies grave difficulty in managing patients with HCV reinfection due to the impossibility of both knowing whether it is a reinfection or a relapse and carrying out resistance studies to define adequate HCV therapy, especially in HIV-positive patients, whose predominant genotype in Colombia is genotype 4. Thus, it is vital to perform the baseline assessment of the genotype before starting DAA to better guide treatment in those patients with clearly identifiable risk factors.
Testing to identify resistance-associated mutations
One of the most intense debates in the pharmacological management of hepatitis C is the use of sequencing to identify resistance-associated mutations before treatment; however, access to tests is limited, and there is no consensus on test techniques, interpretation, and reporting. Highly effective treatments are currently available in cases of HCV infection with pre-existing resistance-associated mutations or substitutions (RAS). Therefore, many scientific societies do not recommend routine testing to identify RAS before treatment in DAA naïve people. However, the American Association for the Study of Liver Diseases recommends the RAS to identify the Y93H substitution in NS5A in previously treated or cirrhotic patients infected with HCV genotype 3 in whom it is intended to use sofosbuvir/velpatasvir (regimen available in Colombia). RAS testing is suggested for the intention to treat with elbasvir/grazoprevir and HCV genotype 1a infection, regardless of whether the patient has had prior treatment. Lastly, the AASLD guidelines recommend requesting RAS in patients previously treated and infected with genotype 1a, in whom it is intended to start ledipasvir/sofosbuvir52,53.
Moreover, the retreatment of patients who have failed after a regimen should be guided by knowledge of which drugs were given in previous treatment courses if resistance testing is not available or, if resistance testing is performed, by the response according to the observed resistance profile. Therefore, when available, RAS tests should guide the individualized choice of retreatment regimens, mainly if NS5A inhibitors were previously used14.
In the case of reinfection, no studies recommend the use or not of molecular resistance tests; however, it is currently believed that they should have the same indications previously listed.
Clinical case
We present the case of a 37-year-old male patient with HIV infection being managed with lopinavir/ritonavir and abacavir. On physical examination, slight hepatomegaly was noted, with no additional stigmata of chronic liver disease. The liver biochemical profile revealed an alteration and positive anti-HCV, so further tests were requested to establish the diagnosis. HCV infection, genotype 1, and subgenotype 1a were confirmed. In addition, serological markers were detected that demonstrated a previous hepatitis B virus (HBV) infection. Besides, the liver ultrasound did not show signs of chronic liver disease, and the FIB-4 did not suggest advanced fibrosis (Table 1).
Table 1 Paraclinical results of the patient
| Serum biochemistry | Result |
|---|---|
| Alkaline phosphatase | 113 UI/L (44-147 UI/L) |
| Total bilirubin | 1.67 mg/dL (0.1-1.2 mg/dL) |
| Direct bilirubin | 0.64 mg/dL (< 0.3 mg/dL) |
| AST | 27 U/L (0-35 UI/L) |
| ALT | 56 U/L (0-45 UI/L) |
| GGT | 31 (0-30 UI/L) |
| HBsAg | Negative |
| Anti-Hbc | Positive |
| Anti-Hbs | Positive |
| FIB-4 | 0.48 |
| Anti-HCV | Positive |
| HCV RNA | 254,287 UI/mL |
| HCV genotype | Genotype 1, subgenotype 1a |
ALT: alanine aminotransferase; anti-Hbc: hepatitis B virus core antibody; anti-Hbs: hepatitis B virus surface antibody; AST: aspartate aminotransferase; FIB-4: fibrosis index 4; GGT: gamma-glutamyl transferase; HBsAg: hepatitis B surface antigen. Source: The authors.
Management was ordered with the pan-genotypic regimen for hepatitis C (sofosbuvir, an NS5B inhibitor, and velpatasvir, an NS5A inhibitor) for 12 weeks. The viral load at the end of the treatment was undetectable (<12 IU/mL), so SVR was confirmed. Additionally, serum levels in the normal range of liver enzymes (alanine aminotransferase [ALT]: 12 U/L; aspartate aminotransferase [AST]: 18 U/L) were demonstrated.
Three months later, the patient attended the follow-up appointment. According to the follow-up exams, viral load (HCV RNA: 8,354 IU/mL) was detected, for which the patient was questioned about possible risk behaviors.
After a thorough anamnesis, the patient reported unprotected sexual intercourse on five occasions with multiple partners. Therefore, genotyping was ordered, proving HCV genotype 4 infections. With this finding, we confirmed that the patient had HCV reinfection and prescribed a new treatment regimen with sofosbuvir and velpatasvir for 84 days and subsequent confirmation of SVR12.
Conclusion
We presented the case of a patient with HIV infection with an alteration in the liver profile and a diagnosis of HCV genotype 1, subgenotype 1a infection. The patient received DAA treatment, after which viral clearance was confirmed at 12 weeks of treatment. Three months later, reinfection was suspected due to elevated viral load and altered liver biochemistry, confirming HCV genotype 4 reinfection.
HCV reinfection is expected, mainly in MSM, HIV-infected individuals, and people with multiple sexual partners. Evidence and studies show the importance of being alert to the possibility of reinfection, so it is appropriate to perform HCV genotyping before starting treatment, even in cases with a pan-genotypic DAA regime. It is imperative to remember and insist on the caution that must be used to reduce risky behaviors. Effective treatment of HCV and consequent viral clearance do not induce a specific adaptive immune response against other HCV genotypes and, therefore, do not eliminate susceptibility to infection by the other seven HCV genotypes. Continuous education and multidisciplinary support are critical for these patients.











 texto em
texto em