Introduction
Liver transplantation is the therapy of choice in advanced or terminal stages of liver disease, acute liver failure with poor prognostic indicators, or primary liver tumors when the patient meets the criteria according to multidisciplinary pre-transplant analyses. The number of patients on the waiting list for organ transplants is increasing, but the availability of donors is insufficient. This outlook has favored organ selection from donors with minor incompatibilities1, in addition to the development of immunosuppressive therapy and the prognosis of patients.
Liver transplantation is routinely performed with ABO system blood group compatibility (identical ABO). ABO is defined as compatible (e.g., type O donor to type B recipient), identical (same group), or incompatible (different groups, for example, type A donor to type B recipient); in the donor-recipient pairing, the Rh is not taken into account2. Only in emergent cases is liver transplantation performed with group incompatibility since survival is lower when transplanted with an incompatible ABO group, and there is a greater risk of complications and graft loss due to antibody-mediated rejection. In such cases, a different therapeutic approach is required at centers experienced in liver transplantation with ABO incompatibility3.
In any transplanted tissue, viable B-cell clones and plasma cells transfer from the donor into the recipient’s circulation. Those “passenger” lymphocytes that survive and proliferate escape immunological surveillance due to the immunosuppression to which the recipient is subjected. In other words, there is no rejection of lymphoid clones, so when exposed to the antigen in the recipient’s red blood cells, anti-isohemagglutinin or anti-Rh antibodies are produced, causing immune-mediated hemolysis4.
The risk of hemolysis increases depending on the lymphocyte mass, and it is more common in heart and lung transplantation, followed by liver and kidney transplantation5. When these lymphocytes proliferate, they create an immune response, which can be primary or secondary, with the onset of hemolysis occurring in the first days to weeks after transplantation6.
Passenger lymphocyte syndrome (PLS) is generally associated with mismatched ABO transplants. In other cases, it may occur in patients who receive an identical ABO organ, but there is a mismatch in minor red blood cell antigens between the donor and recipient. Liver transplantation has frequently reported antibodies against Rh antigens (D, C, c, E, e, and V) and, sometimes, alloantibodies against Jk, Kp, Fy, and M antigens, with which hemolytic reaction may happen7,8.
Below is the case of a patient with cirrhosis secondary to metabolic-associated fatty liver disease (MAFLD) with an O/Rh(D) positive blood type who received a liver transplant from an O/Rh(D) negative donor. During the postoperative period, he presented with immune hemolytic anemia due to anti-D antierythrocyte antibodies due to the donor’s prior sensitization. It is a little suspected cause of acute autoimmune hemolytic anemia in the weeks following the transplant; therefore, given the increase in the number of procedures worldwide, it should always be considered.
Case presentation
We present the case of a 54-year-old man with a history of hypertension, diabetes mellitus, and cirrhosis secondary to MAFLD, Child-Pugh B stage, MELD-Na (Model for End-Stage Liver Disease) score of 18 points, with multiple hospitalizations for hepatic encephalopathy and criteria for transplantation. On prior examination, an O/Rh(D) positive blood typing stands out, with negative irregular antibody screening and negative antiglobulin test (direct Coombs). Orthotopic liver transplantation was performed from a male O/Rh(D) negative donor with positive anti-D and anti-C erythrocyte antibodies due to previous sensitization in an unknown context. The ischemia time was five hours; there were no complications during the procedure or red blood cell transfusion requirement in the peri- or postoperative period. During the postoperative period, the patient required vasoactive support for a few hours, early extubation was performed, and elevated aminotransferases with an early cytolysis peak (aspartate aminotransferase [AST]: 588 U/L and alanine aminotransferase [ALT]: 413 U/L) were detected. Early immunosuppressive therapy with methylprednisolone and tacrolimus on day one was indicated. The ultrasound and Doppler follow-ups did not show any alterations. He was discharged on the fifth day after the intervention with tacrolimus at 0.1 mg/kg, prednisolone, and valganciclovir prophylaxis due to positive antibodies for cytomegalovirus in the donor and recipient, in addition to trimethoprim/sulfamethoxazole as prophylaxis against Pneumocystis jirovecii.
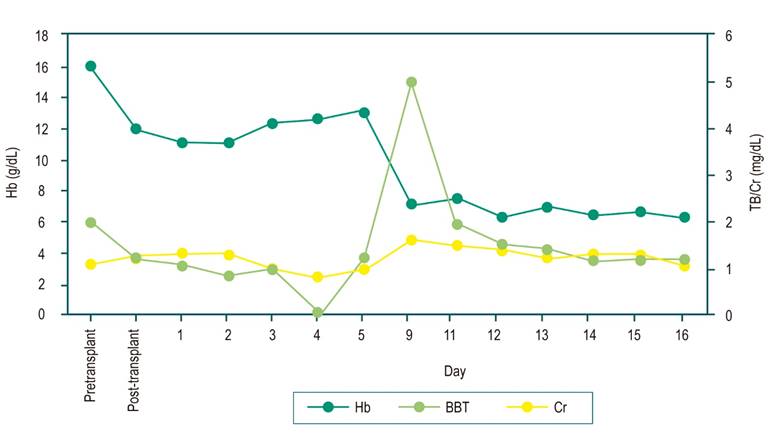
On the ninth day post-transplant, the patient attended the emergency room because he had jaundice, reddish urine, asthenia, adynamia, and hyporexia. The admission laboratory tests (Figure 1) documented severe anemia (7.1 g/dL, reference: 13-18 g/dL), hyperbilirubinemia at the expense of indirect bilirubin (total bilirubin [TB]: 5.01 mg/dL [0.2-1.2 mg/dL], indirect bilirubin [IB]: 3.63 mg/dL), elevated lactic dehydrogenase (531 U/L), consumed haptoglobin (<5 mg/dL), and positive direct antiglobulin test (Coombs) with evidence of anti-D alloantibody and renal compromise with elevated creatinine (Cr) (1.6 mg/dL). Abdominal ultrasound and portal Doppler were normal. Tacrolimus levels were in the normal range. A clinical manifestation related to passenger lymphocyte syndrome was considered, thus indicating management with intravenous fluids and transfusion of two O-negative RBC units associated with pulses of methylprednisolone 500 mg daily for three days. Hemoglobin (Hb) stabilization, hemolysis parameter control, and renal function improvement were achieved, and immunosuppressive management with prednisone at 1 mg/kg/day associated with tacrolimus was continued.
Outpatient follow-up has been carried out in the transplant unit without recurrence of the hemolytic process. The dose of steroids was gradually reduced, and tacrolimus levels have been maintained at targets with hepatocellular structure and function laboratory tests in the normal range: AST: 17 U/L, ALT: 12 U/L, gamma-glutamyl transferase (GGT): 13 U/L, BT: 0.50 mg/dL, IB: 0.32 mg/dL, direct bilirubin (DB): 0.18 mg/dL, alkaline phosphatase: 74 U/L, albumin: 3.39 g/dL, Hb: 15.2 g/dL, and tacrolimus: 6.8 ng/mL.
Discussion
In the first 15 days after liver transplantation, anemia has different etiologies, such as bleeding, sepsis, medications, rejection, or hemolysis. The latter is a significant cause of anemia in this period and may derive from drugs, transfusions, hypersplenism, graft-versus-host disease, or incompatibility of the antigenic systems between donor and recipient9. In turn, the findings of jaundice and anemia make it necessary to rule out arterial or venous thrombosis, vascular stenosis, biliary complications, or sepsis10.
Passenger lymphocyte syndrome (PLS) is a type of anemia that occurs after transplantation11. It is considered a subtype of graft-versus-host disease that affects patients in the first month, generally three to 24 days after solid organ or hematopoietic stem cell transplantation6. In the case described, the compromise occurred on the ninth day.
The incidence of PLS in solid organs depends on their lymphocyte mass, which is why it is more common in lung and heart transplants (70%), followed by liver and kidney transplants (40% and 17%, respectively)5. Moreover, 50% of patients develop antibodies in cases of incompatibility, but not all show evidence of hemolysis. In Ramsey’s series in 1991, 12 cases of PLS occurred out of 1,200 liver transplants, for a prevalence of 1%12. In 2015, in a series of 1,217 liver transplants, Romero et al. reported 56 cases of ABO group incompatibility, of which 17.9% developed PLS, and of 147 patients with Rh incompatibility, 1.4% had PLS8.
The viable B cells transferred in the donor graft proliferate and cause the entity due to the production of antibodies against specific antigens of the recipient’s red blood cells, which produces a primary or secondary immune response7. This entity is a consequence of minor ABO or Rh system incompatibility and rarely of other minor antigenic systems of red blood cells (D, C, E, Jk, Kell, Fy, and M)13. Serum antibodies are predominantly of the immunoglobulin G (IgG) type, but in some cases, they are of the immunoglobulin M (IgM) type13. These tend to disappear within 3-6 months, except for anti-D, which can persist for up to a year14.
Risk factors for PLS include previous sensitization by transfusion or pregnancy, group O donor versus a group A or B recipient, or treatment with cyclosporine15. In the case described, the recipient was O/Rh(D) positive, but the donor was O/Rh(D) negative, indicating that the latter was sensitized in an unknown context because of documentation of anti-D and anti-C in his serum and the detection of the anti-D alloantibody in the previously absent red blood cells of the recipient. Therefore, the cause of the hemolysis is attributed to the anti-D antibody.
Generally, the manifestation is mild and self-limited with spontaneous remission, although it can sometimes be severe16. In other cases, it may be subclinical and an incidental finding in follow-up laboratory tests or superimposed on perioperative complications. The severity will depend on three factors: the amount of transplanted lymphoid tissue, the isohemagglutinin titer of the donor’s red blood cells, and the kinetics of post-transplant antibody production17. Non-hemolytic variants, serological reactions, and even severe events such as liver rejection, hypotension, disseminated intravascular coagulation, and renal or multiorgan failure have been reported5. In the present case, the patient developed hemolytic anemia and acute kidney injury in KDIGO 1 (Kidney Disease: Improving Global Outcomes) stage.
Laboratory findings are consistent with acute hemolytic anemia, decreased haptoglobin, increased lactic dehydrogenase, increased reticulocytes, hyperbilirubinemia at the expense of indirect bilirubin, and peripheral blood smear with spherocytes, polychromasia, agglutination, or nucleation of red blood cells18. The direct antiglobulin test becomes positive in the transplanted patient, which is a good indicator in detecting antibodies derived from the donor’s lymphocytes6. In our case, the hemolysis features described with a strongly positive direct antiglobulin test, previously negative in the pre-transplant assessment, were present.
Currently, there is no specific management but support measures and transfusion of red blood cells. It should be clarified that, for the latter, the recommendation is to transfuse units compatible with the donor or with negative antigens due to the risk of perpetuating hemolysis6. In the presence of bidirectional ABO group incompatibility, group O RBC units compatible with the donor and recipient should be transfused. Negative units should be used in the case of antibodies against minor antigens. For this reason, the patient received irradiated and leukoreduced O-negative RBC units.
Immunosuppressive or immunomodulatory therapy may also be required to induce remission in antibody production when hemolysis is severe or persistent. Administering glucocorticoids, whose dose is suggested to be increased to 1 mg/kg/day, with intravenous steroid pulses (250 to 500 mg of methylprednisolone) is the most widely used treatment and usually sufficient to resolve the clinical picture. The use of rituximab, intravenous immunoglobulin, or plasmapheresis has also been described in some cases19. Most therapeutic supports are based on observational studies or case reports. Clinical studies are required to define a practical approach, as well as the type and duration of treatment. In the case presented, there was a favorable clinical evolution and response with fluid intake, red blood cell transfusion, and methylprednisolone pulses. In case of recurrence, one can opt for the depletion of B cells and plasma cells using rituximab or plasmapheresis based on the pathogenic principle to remove immune complexes, complement components, and antibodies from the circulation6.
PLS antibodies can persist at detectable levels from 12 days to 851 days post-transplant, so outpatient monitoring of hemolytic variables is essential20. The patient is being followed up in the transplant unit through a complete blood count, liver tests, hemolysis, and normal tacrolimus levels, with no evidence of alterations one year after the transplant, an unaltered liver profile, stable Hb, and immunosuppression levels according to the targets.
Conclusion
The diagnosis of passenger lymphocyte syndrome is challenging. It should be suspected in the presence of acute anemia in the first days and weeks of a liver transplant, especially if there is either group or Rh incompatibility or if there was positive antibody screening in the donor. Clinical and laboratory findings suggesting hemolysis, a positive direct antiglobulin test, serologic evidence of antibodies to red cell antigens, and the absence of another etiology of hemolysis favor the diagnosis. Management is defined according to the clinical manifestation. Most cases are resolved with support measures, blood transfusion, and, in some specific circumstances, steroids or other therapies. The patient’s follow-up in the post-transplant period must be conducted with hemolytic parameters.











 texto en
texto en