Introduction
On December 31, 2019, hospitals in Wuhan, Hubei, China, reported a cluster of pneumonia cases of unknown cause at the time. On January 7, 2020, researchers isolated a new coronavirus from patients with pneumonia and named it SARS-CoV-2. On January 30, the World Health Organization (WHO) declared COVID-19 (as coronavirus disease 2019 has been known since February 11) a public health emergency of international concern that would later be recognized as a pandemic1,2. Over time, a broad association with extraintestinal manifestations has also been found3. Among them, there is a significant increase in the incidence of thrombotic events; notably, portal vein thrombosis (PVT) has been reported in patients with no other potential causes of thrombophilia other than a current or recent SARS-CoV-2 infection, as in the case we present4.
There are various immunosuppression schemes after orthoptic liver transplantation5. One of the most frequently used is induction with corticosteroids and maintenance with tacrolimus. Nevertheless, it is necessary to know that this calcineurin inhibitor exhibits, in addition to numerous drug interactions, several adverse effects, including diarrhea, persistent ascites, and acute kidney injury. Therefore, in the liver transplant postoperative period, its serum level should be assessed to consider its adverse effects as a differential diagnosis of these complications6-8.
Case presentation
We present the case of a 48-year-old male patient with decompensated liver cirrhosis (Child-Pugh class B and Model for End-stage Liver Disease [MELD] 15) diagnosed one year ago, secondary to non-alcoholic steatohepatitis (NASH). During this period, he had multiple episodes of encephalopathy, ascites, and hypertensive portal bleeding, requiring hospitalization and ligation of esophageal varices. He was admitted to the Imbanaco Clinic for an orthoptic liver transplant because he had a compatible cadaveric donor. He was scheduled for the procedure 40 days before; however, it was not performed at the time as he had a positive polymerase chain reaction (PCR) test for SARS-CoV-2. Within the initial tests, the PCR for SARS-CoV-2 was again positive. The infections committee authorized the transplant considering that the positive sample corresponded to residual RNA in the absence of active infectious viral particles.
The liver transplant was performed with cold ischemia for five hours and 45 minutes and hot ischemia for 40 minutes, with vasopressor support, intraoperative bleeding of 1.7 liters, and transfusion of two RBC units, six cryoprecipitates, and a platelet pool. He was admitted to the intensive care unit (ICU) with no ventilatory support, vasoactive support with norepinephrine, and adequate urinary output. According to the protocol, immunosuppression was started with methylprednisolone at a dose of 200 mg/day in a tapering scheme as induction, and on the second day, tacrolimus XL at 7 mg/day for maintenance. After a hospital stay with no complications, preserved renal (creatinine 0.63 mg/dL, blood urea nitrogen 5.2 mg/dL) and liver function, hemodynamic stability, no respiratory symptoms or elevation of acute inflammatory reactants, and Doppler ultrasound of the transplanted liver within normal limits, he was discharged on the eighth post-transplant day with a prescription of tacrolimus XL at 9 mg/day, prednisolone 5 mg/day, and valganciclovir 450 mg every 12 hours.
One month after the transplant, the patient was readmitted due to a 10-day history of abdominal distension, increased frequency of liquid stools, no fever, and normal diuresis. On admission, he had abdominal pain and was normotensive, tachycardic, tachypneic, and euthermic. A portal Doppler revealed much ascitic fluid, and the transplanted liver appeared normal. The patient required an evacuatory paracentesis with albumin replacement at 8 g per liter; the administration of diuretics and the supply of intravenous fluids were started. Infectious etiologies were ruled out during the stay, with a viral load for cytomegalovirus, PCR for Clostridium difficile, and negative blood, urine, and stool cultures. Renal function showed a progressive deterioration with an initial creatinine of 2.33 mg/dL up to 4.04 on the fourth day of hospitalization. Tacrolimus levels were obtained at 10.7 ng/mL, so we temporarily suspended the drug. The following day, stools decreased to three in 24 hours and creatinine to 2.4 mg/dL, reaching normal ranges on the sixth day of hospitalization.
Sixty days after the transplant, the patient presented with diarrhea and ascites. Portal Doppler showed portal and suprahepatic veins thrombosis, confirmed by abdominal CT angiography (Figure 1). Anticoagulation was started, and the patient received approval for liver retransplantation. Tests to rule out thrombophilia were negative. Without a cause of the thrombotic event, a history of recent SARS-CoV-2 infection suggests a potential etiology.
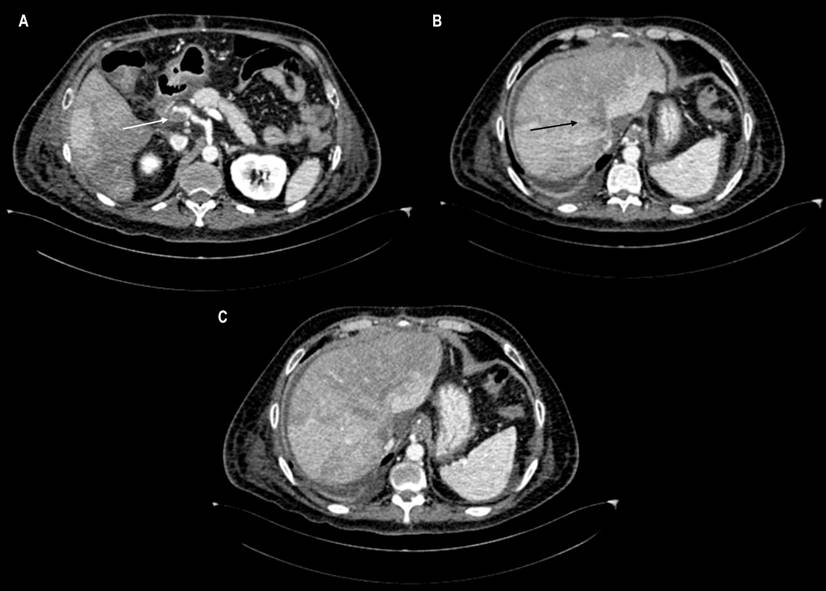
Figure 1 Computerized axial tomography (CT) of the abdomen. A. The axial plane reconstruction shows a thrombus with subtotal obstruction in the portal vein (white arrow). B. Total occlusion in the suprahepatic veins (black arrow), making up the diagnosis of Budd-Chiari syndrome. C. Hepatic areas of heterogeneous enhancement are a characteristic pattern of this syndrome.
Discussion
It has been described that the risk of contagion in patients asymptomatic or with mild symptoms is low from day 7. Wölfel et al. virologically evaluated nine patients with mild COVID-19 and found that the virus replication rate decreases from day five, and there is a 5% probability of continuing beyond day 79. The Centers for Disease Control and Prevention (CDC) recommends discontinuing isolation in patients with mild or moderate COVID-19 ten days after symptom onset, fever resolution for at least 24 hours without fever-reducing medications, and improvement of other symptoms. For asymptomatic patients, it should be ten days after their first positive PCR for SARS-CoV-210.
Regarding the indication for transplantation, there are reports of solid organ transplantation groups with different indications for surgery, with restrictions based on the decisions of the multidisciplinary team. Rouphael et al. published the case of a successful liver orthoptic transplantation in a patient with acute liver failure secondary to drug poisoning. The procedure was performed despite a positive initial PCR for SARS-CoV-2 after a risk exposure 6-10 weeks prior11. Some transplant groups suggest that patients be listed as active if six weeks have passed since they had an initial positive test for COVID-19 with at least four weeks without symptoms and that they not be retested with another PCR before transplantation if they are within three months of the initial positive test12.
We present the case of a patient with a history of decompensated liver cirrhosis who was admitted for orthoptic liver transplantation; however, at first, it was not carried out because the PCR for SARS-CoV-2 was positive, despite not having respiratory symptoms. Forty days later, the patient continued to have a positive PCR. In this case, the infections committee regarded it as the persistence of non-infectious viral particles and authorized the orthoptic liver transplant.
It is speculated that COVID-19 has multiple pathophysiological mechanisms that trigger involvement in solid organs, including the liver. The vascular damage would be mediated by an altered coagulation process and the deterioration of the blood circulation or the endothelium. In Bergamo, Sonzogni et al. evaluated postmortem the histological characteristics of 48 liver samples in 47 patients positive for COVID-19 who died of severe respiratory failure with no history, signs, or symptoms of liver disease until the hospital stay. All presented with some degree of vascular thrombosis: partial portal (24), total portal (11), incomplete sinusoidal (7), and complete sinusoidal (6). In addition, variable degrees of phlebosclerosis, herniation, and portal fibrosis were reported13.
A high incidence of thrombotic complications has been reported in critically ill patients with COVID-19. Klok et al. studied 184 patients with COVID-19 admitted to the ICU in the Netherlands to look for the composite outcome of ischemic cerebrovascular accident (CVA), myocardial infarction, or systemic arterial embolism. There was a mortality of 22%, thrombotic complications in 75 cases, 65 pulmonary thromboembolisms, one deep vein thrombosis, two cases of upper extremity thrombosis, five patients with ischemic stroke, and two with systemic arterial emboli. Patients on chronic anticoagulation therapy on admission were associated with a lower risk of the composite outcome (hazard ratio [HR] 0.29; 95% confidence interval [CI] 0.091-0.92), whereas patients with thrombotic complications had a higher risk of death from any cause (HR 5.4; 95% CI 2.4-12)3.
It has been shown how PVT may occur in patients without other possible identifiable causes. Borazjani reported the case of a 23-year-old asthmatic man with coronavirus pneumonia who presented with acute generalized abdominal pain, mild ascites, and PVT. Routine laboratory data regarding secondary causes of PVT were normal. In our patient, partial PVT and thrombosis of the suprahepatic veins were found, ruling out secondary causes of thrombosis. Progressive hepatomegaly occurred, which is why he underwent an orthoptic liver transplant again.
Apart from the history of recent recovery from SARS-CoV-2 infection, our patient had adverse effects caused by immunosuppression, specifically tacrolimus. He had diarrhea and prerenal acute kidney injury associated with hypovolemia one month after transplantation. When tacrolimus was discontinued, diarrhea stopped, and there was a progressive decrease in nitrogen concentrations until reaching normal levels. According to the American Society of Transplantation (AST) Infectious Diseases Community of Practice guidelines, after ruling out infectious etiologies of diarrhea in solid organ transplant recipients, it is necessary to consider reducing the dose of immunosuppressants and assessing the response8. In addition to episodes of vomiting or diarrhea, it has also been described that calcineurin inhibitor toxicity by itself may cause acute kidney injury post-transplantation, which is why drug levels should be measured and the dose adjusted according to the desired concentration6. Rodríguez et al. revealed that obtaining a trough concentration of tacrolimus between 6 and 10 ng/mL during the first 4-6 weeks after orthoptic liver transplantation reduces renal failure without simultaneously increasing the risk of acute cellular rejection. Then a progressive dose reduction should be achieved to reach a tacrolimus serum level between 4 ng/mL and 8 ng/mL in the long term14.
Conclusion
There are recommendations from transplant groups regarding the isolation and surgical indications of the patient with COVID-19; however, these must be defined through institutional protocols and the infections committee. Once transplanted, multiple complications may occur in the patient who recently recovered from a SARS-CoV-2 infection; extrapulmonary complications with a thrombotic component must be recognized early. Furthermore, knowing the interactions and recognizing early the adverse effects of immunosuppression makes it possible to anticipate dose modifications to attain a safe and effective plateau concentration.











 texto em
texto em 



