Introduction
Solid pseudopapillary tumor of the pancreas is also called Frantz’s tumor, solid and papillary tumor, solid-cystic tumor, papillary cystic tumor, or solid and papillary epithelial neoplasm1. Lichtenstein was the first to report this entity in 19342, but Virginia Kneeland Frantz described its pathology in 19593, and Hamoudi et al. explained its electron microscopy characteristics in 19704. The World Health Organization (WHO) classified it as a solid pseudopapillary neoplasm (SPPN) in 19965. The prevalence has soared in recent years because of increased incidental detection of cystic pancreatic lesions on imaging such as computed tomography (CT) and magnetic resonance imaging (MRI) of the abdomen to study other causes6. The number of cases reported in the literature has increased seven times in the last two decades6.
The present study reports five cases of SPPN and reviews its clinical, radiological, histological, treatment, and prognosis aspects.
Materials and methods
A series of five cases is reported, four women and one man between 16 and 36 years old, with a diagnosis of SPPN. The patients were diagnosed and treated between May 2017 and April 2020 at a tertiary care hospital in Bogotá, Colombia. The majority presented with similar symptoms, consisting of abdominal pain and distension. Two of the five patients had nausea, emesis, and fever; one had low back pain, and another exhibited weight loss. The tumor size and location were variable at the level of the head (1), neck (1), and body and tail (3) of the pancreas, with a distal predominance. Four spleen-preserving laparoscopic distal pancreatectomies and one open pancreatoduodenectomy were performed. For the literature review, we searched PubMed and Google Scholar; articles in Spanish and English were included, and no article was excluded due to the publication date.
Case presentation
Case 1
A 27-year-old woman attended the emergency department due to diffuse and intermittent abdominal pain of two months associated with abdominal distension. An ultrasound and CT scan of the abdomen with a contrast medium revealed a cystic mass in the neck of the pancreas (3.6 cm x 3.6 cm x 3.5 cm) with high suspicion of a solid pseudopapillary tumor of the pancreas. Tumor markers CA 19-9 and carcinoembryonic antigen were negative. A biliopancreatic endoscopic ultrasound (EUS) was requested, which reported a lesion in the body of the pancreas distal to the splenoportal confluence, with elastography suggestive of a malignant lesion with a strain ratio of 180 (normal < 25).
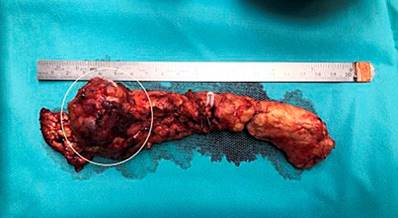
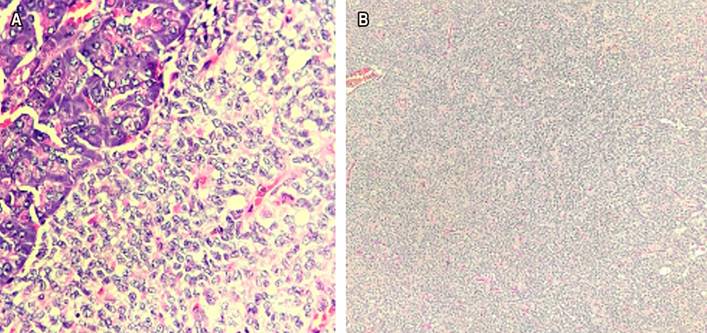
Distal pancreatectomy was conducted by spleen-preserving laparoscopy, finding an approximately 5 cm tumor in the neck of the pancreas (Figure 1) that did not compromise the vascular structures and lymphadenopathy on the hepatic artery, which was resected. The histopathological report confirmed the diagnosis of solid pseudopapillary neoplasm (Figure 2) with negative lymphadenopathy for the tumor. There were no postoperative complications. Four months after the intervention, the patient’s condition was adequate, with modulation of abdominal pain. A follow-up CT scan of the abdomen with a contrast medium was performed with post-surgical findings of distal pancreatectomy and old splenic infarction.
Case 2
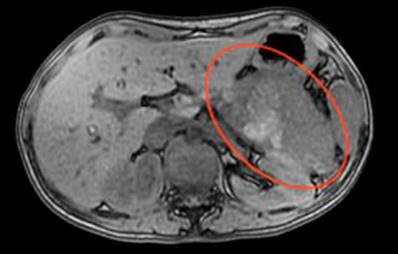
A 23-year-old female patient consulted the emergency department due to abdominal pain of a month’s evolution associated with febrile episodes of 38 ºC, multiple emetic episodes, weight loss, and dysmenorrhea, with extra-institutional studies that ruled out an infectious focus. The CT scan of the abdomen with a contrast medium was consistent with a neoplastic mass in the body of the pancreas and compression of the gastric chamber. On admission, she had a hemoglobin of 9.4 g/dL and a platelet count of 608,000. An MRI of the abdomen (Figure 3) was requested with a protocol for the pancreas. It reported a mass in the tail measuring 10 cm x 8 cm with an anterior cystic component and a solid posterolateral component, a compressive effect on the viscera, and a poor view of the splenic vein, which suggested thrombosis. The splenoportal Doppler report did not document vascular alterations.
She was scheduled for distal pancreatectomy with laparoscopic splenectomy. During surgery, a 15-cm hypervascularized mass was resected. It adhered to the transverse mesocolon with fibrosis of the posterior face of the stomach in contact with the splenic artery and vein, which were dissected and preserved. There were adenopathies in the celiac trunk, which were resected and sent for frozen biopsy and did not report malignancy. A splenectomy was not conducted, and four units of packed red blood cells were transfused. In the postoperative period, she presented with anemia compared to the admission values (7.6 g/dL) and hypotension, for which she required additional transfusion support.
The patient was discharged and readmitted eight days later due to blood content greater than 300 mL in the drainage. A CT scan of the abdomen was performed with expected post-surgical changes, and a small amount of free fluid on the surgical bed was considered normal. The diagnosis of solid pseudopapillary neoplasm was confirmed with the histopathological report. At 16 months after the intervention, the patient was asymptomatic; a follow-up CT scan of the abdomen did not show a tumor relapse.
Case 3
A 36-year-old woman with a history of arterial hypertension was scheduled for distal pancreatectomy and laparoscopic cholecystectomy due to a solid pseudopapillary tumor of the tail of the pancreas and symptomatic cholelithiasis. During surgery, an 8 cm lesion was found near the body and tail of the pancreas with cystic content dependent on the main duct. It was resected with spleen preservation without complications. She had complex postoperative management due to pain, emetic episodes, and infection of the surgical site, for which analgesic, antiemetic, and antibiotic treatment was provided. The histopathological diagnosis (Figure 4) confirmed a solid pseudopapillary neoplasm. Two months after surgery, the patient developed a pancreatic fistula, which required percutaneous drainage with adequate resolution. At the last follow-up, 20 months after surgery, she was found to be asymptomatic.
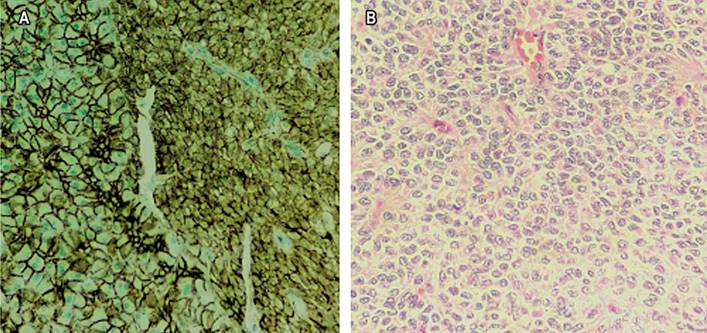
Figure 4 A. Immunohistochemical study for β-catenin shows abnormal nuclear expression in neoplastic cells (right) compared to regular membrane expression in adjacent pancreatic tissue (left). B. Tumor cells display nuclei with finely granular chromatin and irregular nuclear surfaces, some with characteristic longitudinal slits. Source: Authors’ archive.
Case 4
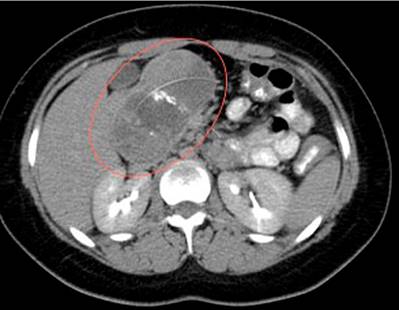
A 16-year-old female patient attended the emergency department for a week-long clinical picture of abdominal pain in the epigastrium, diarrheal stools, and unquantified fever associated with global headache. She had a history of autoimmune thrombocytopenic purpura at age 2. A total abdominal ultrasound revealed a retroperitoneal mass. Subsequently, a CT scan of the abdomen with a contrast medium was performed (Figure 5), which reported a neoplastic-looking focal lesion in the head of the pancreas; a mucinous cystadenoma or neuroendocrine tumor (NET) was considered as a differential diagnosis. Finally, a contrast-enhanced MRI of the abdomen showed a complex-looking mass attached to the head of the pancreas with no signs of invasion into adjacent structures, whose differential diagnoses were complex cystic neoplasm or NET.
We performed an ultrasound-guided percutaneous biopsy, reporting a solid pseudopapillary neoplasm, and a laparotomy pancreaticoduodenectomy with findings of a 9.5 cm x 7 cm solid tumor dependent on the head of the pancreas, distortion of the duodenal anatomy, feeding vessels of the gastroduodenal artery towards the tumor, bile duct of standard caliber, and enlarged periportal lymph nodes. The postoperative period elapsed in the intensive care unit (ICU) with an excellent clinical evolution. She was discharged without complications. Six months after surgery, she presented mild acute pancreatitis that resolved without complications. At the last follow-up, 17 months after surgery, she was found to be asymptomatic.
Case 5
A 21-year-old male patient consulted for a clinical picture of three months of evolution consisting of low back pain with no other associated symptoms. There were no relevant findings on physical examination. With the diagnostic impression of urolithiasis, a CT urogram was performed, revealing a 28 mm x 29 mm bilobed nodular image in the tail of the pancreas with calcifications inside, suggestive of a mucinous-type neuroendocrine (exocrine) tumor. Subsequently, an MRI of the abdomen was conducted with a protocol for the pancreas, noting a solid cystic lesion suggestive of a pseudopapillary tumor. The patient was scheduled for a spleen-preserving laparoscopic distal pancreatectomy. During tumor resection, a solid lesion was found in the tail of the pancreas, approximately 5 cm in diameter, homogeneous, hypervascularized, with irregular edges, which involved the splenic vein and artery, and no evidence of metastatic lesions.
The distal pancreas was dissected with the splenic artery and vein after identifying the superior mesenteric vein. We noted the described tumor, performed the ligation of the splenic vein and artery with distal and proximal Hem-O-Lock®, resected the infiltration of the tumor, and preserved the short gastric vessels and the left gastroepiploic artery. Subsequently, the distal pancreas was resected. The spleen was checked with adequate perfusion without signs of ischemia, infarction, or complications. Pathology diagnosed a solid pseudopapillary neoplasm.
He was readmitted 14 days later due to a six-hour history of epigastric pain radiating to the back, associated with emesis, and no fever. There were no signs of an acute abdomen. On admission, paraclinical tests were requested, showing leukocytosis with neutrophilia, direct hyperbilirubinemia without metabolic acidosis, electrolyte balance, preserved renal function, and unaltered amylase. An intra-abdominal collection was suspected, so broad-spectrum antibiotic and analgesic management was started. A contrast-enhanced CT scan of the abdomen reported a collection of approximately 62 mm x 138 mm x 50 mm in the surgical bed, with images suggesting multiple splenic infarcts and no other alterations. Interventional radiology drained 140 mL of residual hematic fluid with a report of positive amylase. He presented with a pancreatic fistula.
The patient had a good evolution with antibiotic management and drainage of the collection without requiring splenectomy. One month later, a follow-up CT scan of the abdomen was performed with a report of splenic infarcts without changes, distal pancreatectomy, and a residual collection of 33 mL, for which the catheter was withdrawn. Five months after the intervention, the patient’s condition was adequate, and a follow-up CT scan of the abdomen revealed changes in distal pancreatectomy and no evidence of collection.
Results
Four patients underwent laparoscopic distal pancreatectomy without splenectomy, and one underwent laparotomy pancreatoduodenectomy. The tumor was completely and satisfactorily removed from all five patients. No metastasis was found. The tumors were located in the head (1), neck (1), and body and tail (3) of the pancreas, with a distal predominance. The postoperative histopathology report confirmed the diagnosis in all five cases. An ultrasound-guided percutaneous biopsy had been performed on the youngest patient prior to surgical intervention.
Discussion
SPPNs are defined by the fifth edition of the WHO Classification of Tumors of the Digestive System as low-grade malignant tumors composed of loosely cohesive epithelial cells that form solid, pseudopapillary structures and lack a specific line of pancreatic epithelial differentiation7. SPPNs are rare: they represent around 1%-3% of all resected cystic tumors8. They frequently occur in women between the second and third decades of life, and less than 10% are diagnosed in men1. In our series, 80% of SPPNs occurred in women. They were located mainly near the body and tail of the pancreas, although they can also involve the head and neck9, as found in our series. Moreover, 1%-1.8% of SPPNs have an extrapancreatic location, mainly in the retroperitoneum, omentum, mesentery, stomach, duodenum, colon, liver, adrenal gland, ovary, testis, and lung1,10.
It has not been possible to establish which cells are involved in developing SPPN; they seem to derive from pluripotent stem cells of the genital ridges that join the pancreas during embryogenesis11. SPPN has been related to mutations in chromosome 11q, which contains genetic material for forming proteins such as cyclin D1, FLI-1, CD56, and the progesterone receptor1,12. Even though SPPN expresses the progesterone receptor that makes it sensitive to hormones, which could explain its prevalence in women, its role in tumorigenesis has not been proven13. Additionally, there are mutations in exon 3 of the β-catenin gene, through which the Wnt signaling pathway is activated. It is enhanced by the BCL9 gene, which is present in this type of tumor, increasing the transcriptional activity and oncogenesis (Figure 6)1,12,14.
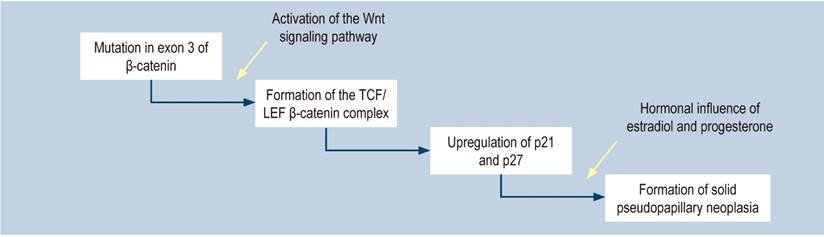
Figure 6 Physiopathology of solid pseudopapillary neoplasm of the pancreas1. Modified from: Lanke G et al. World J Gastrointest Endosc. 2018;10(9):145-155.
SPPN affects pancreatic exocrine tissue, which comprises acinar cells that produce digestive enzymes. They are pyramidal cells with granules surrounded by a membrane containing trypsinogen, chymotrypsinogen, procarboxypeptidase, proelastase, kallikreinogen, and prophospholipases A and B15. SPPNs are usually made up of different elements, such as hemorrhagic areas and calcifications, and feature a fibrous capsule16. Cytoplasmic vacuoles, granule nuclei, and hyaline globules may be present at the cellular level, aiding the diagnosis, as shown in Figure 2A 17,18. Additionally, there is a monomorphic, uniform, small to medium-sized cell population on a clean or hemorrhagic background and papillary structures, as shown in Figure 2B 11. The confirmatory diagnosis is made through immunohistochemical studies that mainly verify the mutation of the β-catenin protein, as shown in Figure 4A 14. The immunohistochemical panel proposed for diagnosing SPPN involves positive markers for β-catenin and CD99 (dot pattern) and negative markers for chromogranin, trypsin, BCL10, and E-cadherin11. EUS-guided fine needle aspiration (FNA), confirmed by immunocytochemistry on the cell block, is helpful for diagnosis, which excludes other pancreatic tumors19. EUS-guided fine needle biopsy (FNB) provides an adequate tissue sample for better diagnostic accuracy1. The histopathological study and immunohistochemistry allow the assessment of differential diagnoses (Table 1).
Table 1 Differential diagnosis of solid pseudopapillary neoplasm of the pancreas1
| Cystic adenoma |
| Cystadenocarcinoma |
| Microcystic adenoma |
| Sarcoma |
| Angiolymphoma |
| Acinar cell cystadenocarcinoma |
Modified from: Lanke G et al. World J Gastrointest Endosc. 2018;10(9):145-155.
This type of tumor usually has non-specific clinical manifestations, most of them caused by compression of the tumor against the normal pancreatic parenchyma (Table 2)1,20. About 15% of patients are asymptomatic20.
Table 2 Clinical manifestation of solid pseudopapillary neoplasm of the pancreas1
| Epigastric and dorsal pain |
| Early satiety |
| Abdominal distension |
| Jaundice |
| Weight loss |
| Nausea and vomiting |
| Palpable abdominal mass |
Modified from: Lanke G et al. World J Gastrointest Endosc. 2018;10(9):145-155.
The imaging studies that can be performed are ultrasonography, CT, and MRI of the abdomen with a contrast medium (Table 3). CT and MRI of the abdomen are about 60% accurate in determining the correct histological diagnosis of cystic lesions of the pancreas. They are also a diagnostic tool that helps to differentiate aggressive lesions from non-aggressive ones in 75% to 90% of cases21. One of the common elements that can be seen in diagnostic images is an encapsulated lesion with solid and cystic components, intramural hemorrhage, fibrous capsule, and, occasionally, calcifications22. SPPN should be considered as the primary differential diagnosis when there is evidence of a large mass at the level of the body or tail of the pancreas, with defined contours, solid and cystic portions, and no internal septa21,23. A study found that, in multislice computed tomography imaging, men generally have smaller lesions with a more significant solid component and calcifications than women24.
Table 3 Findings of solid pseudopapillary neoplasm of the pancreas according to the imaging study21
| EUS | CT | MRI |
|---|---|---|
| Well-defined circumscribed mass with a fibrous capsule | Encapsulated mass with defined contours and a solid or cystic component | A better view of the capsule and tumor extension |
| A mass effect that compresses different structures with high echogenicity | When the tumor has cystic characteristics, the degree of attenuation may vary depending on the extent of the hemorrhagic necrosis contained in the cyst | On T1: A hypointense fibrous capsule with high-intensity internal bleeding |
| Small tumor with a solid component | Tumor with an attenuation coefficient of 20 to 50 Hounsfield units | On T2: Heterogeneous signal intensity |
| Large tumor with a cystic component showing posterior acoustic enhancement | The cystic component varies in size according to the extent of necrosis, blood clots, and necrotic tumor tissue. However, it is not associated with the size of the tumor | The solid portions are isointense or hypointense compared to the pancreatic parenchyma |
| Low echogenicity mass with cystic areas suggestive of hemorrhagic necrosis | Sometimes hyperintense foci are observed in the tumor, which may correspond to cellular debris or areas of hemorrhagic necrosis | |
| With a contrast medium, the heterogeneous peripheral enhancement could be noted in the early arterial phase, and a subsequent heterogeneous filling of the lesion in the portal phase |
Modified from: Tafur A et al. Rev Med. 2017;25(1):70-77.
We should differentiate this tumor from other pancreatic neoplasms because the cornerstone of treatment in these cases is surgical resection, which is curative in most patients25. Five studies compare the surgical approach: minimally invasive surgery versus open intervention with the different types of pancreatic resection depending on the location, pancreatoduodenectomy, central pancreatectomy, distal pancreatectomy, or enucleation (Figure 7)26. A distal pancreatectomy should be performed in tumors involving the body or tail with some vascular involvement27. This type of splenic-sparing surgery is currently preferred since it is associated with less morbidity, fewer infectious complications, a lower incidence of pancreatic fistula, and a shorter hospital stay27-29. In addition, a significant increase in platelet count has been documented after splenectomy, which has been associated with thromboembolic complications30. Finally, spleen preservation has been related to a better general condition in the long term30.

Figure 7 Algorithm to determine the type of pancreatic resection25. Modified from Liu M et al. Pancreatology. 2019;19(5):681-685.
Where possible, one should always try to preserve the spleen, even when the splenic vascular bundle is compromised and must be ligated. Splenic preservation can be achieved by spleen vessel-sparing distal pancreatectomy or the Warshaw surgical technique31. This technique involves ligating the splenic artery and vein, preserving the spleen’s circulation by sparing the short gastric and left gastroepiploic vessels32,33. A meta-analysis that compared the preservation of splenic vessels with the Warshaw technique showed that this technique had a significantly higher incidence of splenic infarction and gastric/epigastric varices, in addition to the fact that more patients with splenic infarction underwent splenectomy. Still, there were no differences between the two procedures regarding pancreatic fistula, morbidity, and hospital stay31. Likewise, splenic preservation using the Warshaw technique is associated with less postoperative morbidity than splenectomy34. Currently, minimally invasive surgery is preferable because it is associated with shorter surgical time and postoperative stay. However, no intraoperative differences are associated with less blood loss, transfusion requirements, postoperative complications, mortality, or surgical resection margins35-39.
In most patients, the disease is localized, and only 9%-15% have a local invasion or metastasis6. Malignancy cannot be easily predicted based on preoperative findings and immunohistochemical patterns40. Deep parenchymal invasion into the surrounding tissue is the most common pathological feature suggesting malignancy41, in addition to lobulated margins and focal discontinuity of the capsule, exophytic growth pattern, solid-cystic component ratio, and Ki-67 index40. A recent meta-analysis confirmed that the risk of malignancy in SPPN increases with the gradual increase of the Ki-67 index42.
The prognosis is generally good. The five-year survival is close to 97%, even in the presence of metastases, which shows that it is a relatively indolent disease compared to other pancreatic neoplasms43. Metastases occur mainly in the liver or peritoneum. The molecular alterations in SPPN with metastases are mutations with CTNNB1 activation and inactivation of epigenetic regulators such as KDM6A, TET1, and BAP144.
Recurrence after surgical resection of non-metastatic SPPN is 2%. The main risk factors for recurrence are being a man (odds ratio [OR]: 1.96), positive lymph nodes (OR: 11.9), R1 margins (OR: 11.1), and lymphovascular invasion (OR: 5, 5), all with p < 0.0545. Additionally, the size of the tumor (> 5 cm) and synchronous metastases have been associated with recurrence46. There is no specific classification system to predict results in SPPN. Although there are many factors, it has not been possible to consolidate a determining element related to recurrence47. Physiopathological similarities between pancreatic NETs and SPPNs have recently been found, with the association of the Ki-67 index as a factor that could reflect recurrence48,49. The WHO introduced the Ki-67 index in 2017, an antigen used to study tumor growth rate for pancreatic NETs47. Tumor grade (Table 4), based on the Ki-67 index in pancreatic NETs, has been incorporated into SPPNs as a critical histopathological test to study tumor growth rates48,49. Zou et al. conducted a study in which they related the tumor grade according to the Ki-67 index and the lesion size with recurrence after tumor resection. The grade based on Ki-67 was higher than that proposed by the American Joint Committee on Cancer with the TNM system to assess recurrence in SPPN42. A multicenter European study that included 149 patients who underwent complete resection of an SPPN found that preoperative EUS-guided FNA did not affect recurrence50.
Table 4 Tumor grade associated with the Ki-67 index47
| Grade 1 | < 3% |
| Grade 2 | 3%-20% |
| Grade 3 | > 20% |
Modified from: Inzani F et al. Endocrinol Metab Clin North Am 2018;47(3): 463-470.
Lastly, an algorithm is presented for the correct diagnostic approach, treatment, and follow-up of patients with SPPN, considering the clinical manifestations and the complementary studies available in each institution (Figure 8)1.

Figure 8 Algorithm for diagnosing, treating, and monitoring SPPN1. NMR: nuclear magnetic resonance; CT: computerized axial tomography. Modified from: Lanke G et al. World J Gastrointest Endosc. 2018;10(9):145-155.
Conclusions
SPPN is a rare, low-grade malignancy that mainly affects young women. The signal symptom may be vague, or the patient may even be asymptomatic, so it is often diagnosed incidentally. Clinical findings, radiological imaging, and cytology constitute the basis of its diagnosis. Surgical resection of the tumor continues to be the mainstay of its treatment. Multiple surgical methods are used to resect these tumors depending on the part of the pancreas affected, its size, and the extent of local invasion.











 text in
text in