Introduction
Autoimmune hepatitis (AIH) is a chronic inflammatory liver disease with a diverse range of clinical manifestations. The disease is characterized by progressive hepatocellular necroinflammatory activity and secondary fibrosis, as initially described by Waldestrom in 1950.1 The pathogenesis of AIH is mediated by an immune reaction against autoantigens of the hepatocyte membrane, a loss of immune tolerance, and an increased risk of developing cirrhosis, hepatocellular carcinoma, and acute liver failure. Traditionally, two types of autoimmune hepatitis have been described, with type 1 accounting for 80% of cases and a female-to-male ratio of 3.6:1.2,3 With a worldwide incidence and prevalence of 1.37 and 17.44 per 100,000 inhabitants, respectively, AIH cannot be considered a rare phenomenon. The current study aims to describe the sociodemographic, clinical, and laboratory characteristics of AIH adult patients treated at a university hospital in Cali, Colombia, as well as the treatments received, their response to them, and relevant outcomes.
Materials and methods
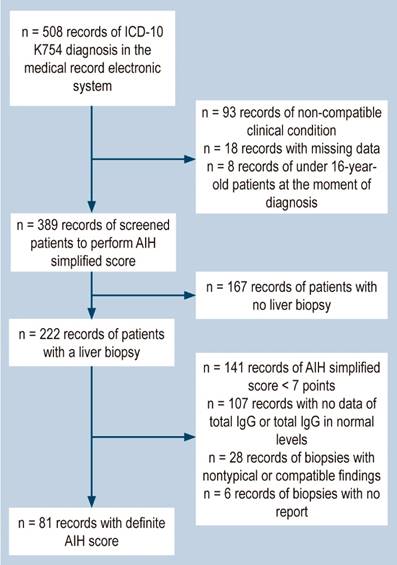
This historical observational group study enrolled patients over the age of 16, of both sexes, who were diagnosed with autoimmune hepatitis (AIH) and treated at Hospital Universitario Fundación Valle del Lili between January 2014 and December 2020, in various clinical settings, including emergency, outpatient, intensive care unit, and inpatient services. We utilized the institutional medical records software system, SAP, to identify patients with an ICD-10 K754 diagnosis reported in their corresponding medical records upon admission, followed by subsequent screening (Figure 1) until finding the ones with a definitive diagnosis of AIH. To achieve this, we used the International Autoimmune Hepatitis Group (IAIHG) scoring system of 2008,4 as specified in Table 1. In cases of overlap syndrome, we included patients with the necessary biochemical characteristics, autoantibody profile, hepatic histological findings, and cholangiographic findings.5 We also gathered demographic, clinical, serological, histological, and treatment variables, as well as outcomes such as cirrhosis or a change in Child-Pugh score, transplantation, and death (related or unrelated to AIH).
Table 1 Simplified criteria for the diagnosis of autoimmune hepatitis
| Variable | Cut-off Point | Punctuation |
|---|---|---|
| ANA or SMA | ≥ 1:40 | 1 |
| ANA or SMA | ≥ 1:80 | 2 |
| Anti-LKM | ≥ 1:40 | 2* |
| Anti-SLA | Positive | 2* |
| IgG Serum | > Upper limit of normal | 1 |
| > 1.10 times the upper limit of normal | 2 | |
| Hepatic histology (evidence of hepatitis as a necessary condition) | Compatible with autoimmune hepatitis | 1 |
| Typical of autoimmune hepatitis | 2 | |
| Absence of viral hepatitis | Yes | 2 |
| ≥ 6 points: Probable autoimmune hepatitis | ||
| ≥ 7 points: Definite autoimmune hepatitis | ||
| Typical histology of autoimmune hepatitis: interphase hepatitis, lymphocytic/plasmacyte infiltrates in portal spaces with lobule extension, emperipolesis, and rosette formation. | ||
| Histology compatible with autoimmune hepatitis: chronic hepatitis with lymphocytic infiltrate without the other typical findings of autoimmune hepatitis. | ||
*The maximum sum for autoantibody points is 2. ANA (antinuclear antibodies), LKM (liver kidney microsomal type-1 antibodies), SLA (antibodies against soluble liver antigen D), and SMA (anti-smooth muscle antibodies). Source: Hennes EM, et al.4
Categorical variables are presented as absolute and relative frequencies, while continuous variables are reported as mean and standard deviation if they follow a normal or mean distribution and interquartile range (IQR) if they do not. The research conducted in this study is in accordance with international agreements on biomedical research set by the Council for International Organizations of Medical Sciences (CIOMS) and Resolution 8430 of 1993 of Colombia. As a risk-free study, informed consent was not required from participants; however, the researchers are dedicated to ensuring the confidentiality and privacy of all study subjects.
Based on the patients’ clinical manifestation at the time of diagnosis, we categorized those who had no symptoms but showed abnormal liver profile test results as “asymptomatic with altered liver biochemistry.”
The following are the clinical manifestations:
Nonspecific symptoms: including asthenia, anorexia, pruritus, weight loss, and abdominal pain with evidence of liver biochemical alteration.
Acute hepatitis: characterized by pain in the right hypochondrium, nausea, jaundice, and a pattern of hepatocellular damage evidenced in laboratory tests.
Hepatic cirrhosis: identified by clinical signs of cirrhosis such as gynecomastia, telangiectasias, palmar erythema, collateral circulation, ascites, and encephalopathy, and biochemical signs such as hypoalbuminemia, thrombocytopenia, and prolongation of prothrombin time.
Acute liver failure: indicated by acute hepatitis symptoms with coagulopathy and the development of encephalopathy in the first 26 weeks after the onset of jaundice, according to the definition of O’Grady et al.6
De novo autoimmune hepatitis: observed in patients who received liver transplantation (without a pre-transplant diagnosis of AIH) and developed AIH in the post-transplant period.
The fibrosis stages of liver biopsy were evaluated using either the METAVIR score or transient elastography, when performed. The fibrosis stage was graded from F0 to F4, with F0 indicating the absence of fibrosis and F4 indicating advanced fibrosis with cirrhosis. Histological features were classified as non-compatible, compatible, or typical of autoimmune hepatitis (AIH). Regarding treatment response, normalization of transaminases and immunoglobulin G (IgG) was considered biochemical remission. Clinical improvement and decrease in transaminases, without reaching normalization, were considered partial remission. Patients who did not experience a reduction of transaminase levels by at least 25% from the initial level when treatment began were classified as non-response. Relapse was defined as the elevation of alanine transaminase (ALT) greater than three times the upper limit of normal and elevation of IgG or worsening of histological findings after achieving remission with pharmacological treatment.
Data management and statistical analysis
The password-protected BD Clinic platform and secure servers were used for consulting, downloading, and storing the collected data. Only individuals affiliated with the study had access to the database. Furthermore, each patient included in the study was assigned a study ID to ensure anonymity.
We randomly sampled 10% of the data to evaluate data quality and compared the typed data to the source documents. We randomly sampled another 10% of the data if inconsistencies were found. If the inconsistencies persisted, we reviewed the entire database.
Results
A total of 81 patients met the inclusion criteria (Figure 1). Of these, 80.2% were women with a median age of 49 (interquartile range [IQR]: 30-61). Autoimmunity comorbidities were present in 28.4% of the patients. Among this subgroup, autoimmune thyroid disease was the most common (14.8%), followed by Sjögren’s syndrome (6.2%), rheumatoid arthritis (3.7%), systemic lupus erythematosus (2.5%), and one case of systemic sclerosis. At the first consultation with the institution, acute hepatitis (43.2%) and biochemical alteration (24.7%) were the most common initial conditions, and there were no cases of acute liver failure. Hepatic cirrhosis, either clinical or diagnosed through non-invasive methods, was the initial condition for 17.3% of patients. By the end of the follow-up period, the percentage had increased to 22.2% of the population, with 72.2% in Child-Pugh stage A. Thirteen patients had the autoimmune hepatitis-primary biliary cirrhosis (AIH-PBC) overlap syndrome (Table 2).
Table 2 Clinical characteristics of patients with autoimmune hepatitis
| Variable | n | % |
|---|---|---|
| Age* | 49 (30-61) | |
| Sex | ||
| - Female | 65 | 80.2 |
| - Male | 16 | 19.8 |
| Concomitant autoimmune diseases | ||
| - None | 58 | 71.6 |
| - Autoimmune thyroid disease | 12 | 14.8 |
| - Sjögren's syndrome | 5 | 6.2 |
| - Rheumatoid arthritis | 3 | 3.7 |
| - Systemic lupus erythematosus | 2 | 2.5 |
| - Systemic sclerosis | 1 | 1.2 |
| Form of initial condition | ||
| - Acute hepatitis | 35 | 43.2 |
| - Asymptomatic, only biochemical alteration | 20 | 24.7 |
| - Cirrhosis | 14 | 17.3 |
| - Nonspecific symptoms | 12 | 14.8 |
| - Acute liver failure | 0 | 0.0 |
| Cirrhosis during the follow-up period | ||
| - No | 63 | 77.8 |
| - Yes | 18 | 22.2 |
| Cirrhosis stage (Child-Pugh score) n = 18 | ||
| - A | 13 | 72.2 |
| - B | 5 | 27.8 |
| - C | 0 | 0.0 |
| Overlap syndrome | ||
| - No | 68 | 84.0 |
| - With PBC | 13 | 16.0 |
PBC: primary biliary cirrhosis. Source: Authors’ own research.
A cohort of 50 patients underwent histological analysis based on typical findings. The evaluation, which was conducted using either histological or elastography methods, revealed a prevalence of 29.6% for fibrosis grades 0 or 1.
The following laboratory parameters were obtained: aspartate aminotransferase (AST) levels of 154 U/L (75.5-711.5), alanine aminotransferase (ALT) levels of 150 U/L (79.5-509), total bilirubin levels of 1.6 mg/dL (0.71-5.0), alkaline phosphatase levels of 162 IU/L (100-280), and total IgG levels of 20 g/L (17.1-28.8). Among the patients, 88.9% had antinuclear antibody positivity equal to or greater than 1:80 dilutions, and 43.2% were positive for ASMA. The initial screening of the three cases evaluated showed no positivity for SLA or LKM antibodies (Table 3).
Table 3 Pathology and laboratory characteristics of patients with autoimmune hepatitis
| Variable | n | % |
|---|---|---|
| Histology (n = 81) | ||
| - Typical findings | 50 | 61.7 |
| - Compatible findings | 31 | 38.3 |
| Degree of fibrosis by biopsy or FibroScan elastography (METAVIR index) | ||
| - Grade 0 | 24 | 29.6 |
| - Grade 1 | 24 | 29.6 |
| - Grade 2 | 10 | 12.3 |
| - Grade 3 | 5 | 6.3 |
| - Grade 4 or established cirrhosis | 18 | 22.2 |
| Laboratory parameters | ||
| - AST (UI/L)* | 154 (75.5-711.5) | |
| - ALT (UI/L)* | 150 (79.5-509) | |
| - Total bilirubin (mg/dL)* | 1.6 (0.71-5.0) | |
| - Alkaline phosphatase (IU/L)* | 162 (100-280) | |
| - Total IgG (gr/L)* | 20 (17.1-28.8) | |
| Antinuclear antibodies (dilutions) | ||
| - Negative | 9 | 11.1 |
| - 1;80 | 19 | 23.5 |
| - Greater than 1;80 | 53 | 65.4 |
| Anti-smooth muscle antibodies (dilutions) | ||
| - Negative | 46 | 56.8 |
| - 1;40 | 15 | 18.5 |
| - 1;80 | 5 | 6.2 |
| - Greater than 1;80 | 6 | 7.4 |
| LKM or SLA antibodies | ||
| - Negative or not performed | 81 | 100.00 |
| - Positive | 0 | 0.00 |
*Median and interquartile range. Source: Authors’ own research.
The most frequently used induction therapy for AIH was a combination of prednisolone and azathioprine, which was also used for maintenance during the follow-up period. However, induction therapy initiation was not reported for 7.4% of patients. Four patients required second-line therapies, specifically mycophenolate mofetil. Of the patients evaluated, 87% met response criteria, and relapses occurred in 13.6% (Table 4). Only one fatal outcome occurred, which was not directly related to the diagnosis of AIH (bacteremia in a patient diagnosed at the age of 26 with Child-Pugh B cirrhosis during prednisolone maintenance therapy). Four patients underwent liver transplantation, and another five patients were on the transplant list during the follow-up period. Nineteen patients experienced a change in Child-Pugh or progression to cirrhosis (Table 5).
Table 4 Treatments of patients with autoimmune hepatitis
| Variable | n | % |
|---|---|---|
| Induction therapy | ||
| - None | 6 | 7.4 |
| - Prednisolone | 10 | 12.3 |
| - Prednisolone + azathioprine | 64 | 79.0 |
| - Other | 1 | 1.2 |
| Response | ||
| - No | 10 | 12.3 |
| - Yes | 71 | 87.7 |
| Relapse | ||
| - No | 70 | 86.42 |
| - Yes | 11 | 13.58 |
| Maintenance therapy | ||
| - Prednisolone | 7 | 8.6 |
| - Azathioprine | 25 | 30.9 |
| - Prednisolone + azathioprine | 36 | 44.4 |
| - Other | 4 | 4.9 |
| - ND | 9 | 11.1 |
| Second-line therapies | ||
| - Not used | 77 | 95.1 |
| - Mycophenolate mofetil | 4 | 4.9 |
ND: No data. Source: Authors’ own research.
Table 5 Outcomes of patients with autoimmune hepatitis
| Variable | n | % |
|---|---|---|
| Death during the follow-up period | ||
| - No | 80 | 98.8 |
| - Yes | 1 | 1.2 |
| Liver transplantation | ||
| - No | 77 | 95.1 |
| - Yes | 4 | 4.9 |
| On the transplant waiting list during the follow-up period | ||
| - No | 76 | 93.8 |
| - Yes | 5 | 6.2 |
| Cirrhosis or change in the initial Child-Pugh to the last assessment | ||
| - No | 62 | 76.5 |
| - Yes | 19 | 23.5 |
Source: Authors’ own research.
Discussion
The incidence of AIH is more prevalent in women, as observed in other studies conducted worldwide7,8 and in Latin America9,10, with a rate above 80%. About 28.4% of cases have at least one autoimmune comorbidity, which is lower than the rate reported by Karakaya et al.11 However, the proportion of autoimmune thyroid disease is similar to that found in related studies12,13, which may occur before or concomitantly with AIH. Acute hepatitis and asymptomatic manifestation were the most common, consistent with previous reports by Czaja14 and Feld.15 Furthermore, approximately 24.7% of cases had alterations in the hepatic biochemical profile, with transaminases being predominant in AIH and alkaline phosphatase in overlap syndromes. This finding is higher than that reported by Díaz et al.9, indicating that the differential diagnosis remains broad despite the absence of treatment.
The clinical course of cirrhosis varies between studies. In our study, it was observed in 17.3% of cases, whereas a Japanese group showed grade 4 fibrosis (in definitive cases) in 8.3%.16 Other studies consistently report that one-third of patients are diagnosed with cirrhosis, without distinguishing between classifications by score.17,18
Clinical guidelines underscore the importance of hepatic histology in confirming diagnoses, staging fibrosis, determining clinical prognosis, and ruling out differential diagnoses. In our study, of the patients who underwent initial screening, 57% underwent histological assessment by laparoscopic or ultrasound-guided biopsy. Of those, 84.7% had findings compatible with or typical of AIH. However, the histological changes were not suggestive for 34 patients who had a biopsy because the definitive report was absent or the sample was inadequate. In addition, only 20.8% of the total screened had a simplified score of seven points or higher to confirm the diagnosis of AIH, indicating the ongoing underdiagnosis in low- to middle-income countries. Although other parameters included in the scores can lead to a diagnosis in accordance with recent guidelines,19,20 we chose to use the definitive score as the inclusion criteria for patients who had a liver biopsy with a report of compatible or typical characteristics. From the initial screening, we obtained 50 patients with probable scores who had a biopsy and 55 patients with probable AIH without a biopsy report. These limitations also impede the accurate and serial assessment of fibrosis, which has implications for mortality.21 The frequency of AIH-PBC overlap is similar to that reported in the literature.22 Based on the Paris criteria,23 patients of Hispanic descent had a higher frequency of overlap than non-Hispanic patients (31% vs. 13%).
Early treatment is crucial as up to 17% of non-treated patients with interface hepatitis to diagnostic histology progress to cirrhosis within five years. Among them, 82% with bridging necrosis progress to cirrhosis and have a high mortality rate of 40%-50%. However, patients who receive appropriate treatment have a life expectancy comparable to those without AIH.24 For the duration of this study’s follow-up period, induction therapy consisted of a combination of prednisolone and azathioprine, which has similar benefits compared to prednisolone monotherapy25 but with a lower incidence of adverse effects such as diabetes, obesity, osteoporosis, hypertension, and emotional lability.27 Furthermore, only one case of pancytopenia due to azathioprine resulting in death was reported.
In terms of maintenance therapy, the data were more varied, with 75.3% of patients using an azathioprine-based regimen (either alone or in combination with low doses of prednisolone) and second-line therapy used in 4 patients (specifically, mycophenolate mofetil). According to the most recent 2019 American Association for the Study of Liver Diseases (AASLD) guidelines,19 there have been no significant changes in pharmacotherapy that could account for a shift in treatment trends in our study. The percentage of relapses was comparable to that of another Colombian study9 (mainly due to abandonment and poor adherence to therapy), with the vast majority occurring in the first 24 months, similar to what has been described by Czaja et al.28 Additional factors related to relapse include the duration of adherent treatment and the length of time of inactive disease prior to drug withdrawal, psychological stress,29 concurrent autoimmune disease, polypharmacy,30 ALT and IgG levels at withdrawal, and prednisolone monotherapy, which have been linked to higher rates of cirrhosis development, death, and the need for a liver transplant.31 These factors must be identified and evaluated during patient follow-up, particularly in countries where accessing treatment and specialized consultations is challenging.
Four patients (4.9%) with definitive AIH underwent a liver transplant, all of whom were under 40 years old. There were no mortality or acute liver failure cases during the follow-up period. The only death in this population was unrelated to AIH and occurred in a cirrhotic patient. Out of the 389 patients in the initial screening, 25 (6.4%) died, with eleven cases related to complications of cirrhosis (four with variceal hemorrhage, four from infections associated with spontaneous bacterial peritonitis [SBP] and bacteremia, one with hepatorenal syndrome, one with acute liver failure, and one with metastatic hepatocarcinoma). Fourteen deaths were not related to AIH, with six associated with bacterial and fungal infections not related to SBP, three related to non-hepatocellular neoplasms, two related to serious coronavirus infections caused by SARS-CoV-2, one due to massive bleeding in the immediate post-liver transplantation period, one due to acute graft dysfunction in less than 48 hours post-transplantation, and one due to non-variceal hemorrhage. Unlike Borssen’s study32, which reported 22% of deaths due to cardiovascular etiology, there were no deaths in this study due to this cause. Three patients died due to early post-transplant complications, with two deaths caused by massive bleeding requiring reoperation and one due to hyperacute graft dysfunction with no emergency indication for retransplantation.
The diagnosis of autoimmune hepatitis alone has been shown to increase the risk of cardiovascular and liver disease, as well as long-term extrahepatic malignancy, based on statistical data.8 Without treatment, the prognosis is poor, with a 50% 5-year survival rate and only 10% survival at ten years, while immunosuppression leads to complete remission in 90% of patients.33 Early diagnosis is beneficial, but detection during youth has been found to be an independent variable associated with incomplete response to treatment.34 However, cirrhosis at the time of diagnosis remains an independent prognostic factor for non-response. This was observed in the German35 and Canadian15 groups, but it was not statistically significant in another study,36 which may be related to geographical and genetic factors in the populations studied. As of December 2020, fifteen patients were on the liver transplant waiting list.
Interestingly, patients who received a definitive score had lower rates of cirrhosis at the beginning of treatment, a high percentage of biochemical response, low relapse rates, and lower mortality. This may be attributed to the use of early histology as a diagnostic tool and treatment guide, as well as the early initiation of immunosuppression in the high percentage of non-cirrhotic patients at the time of diagnosis. Compared to patients who received probable scores, their characteristics and findings were similar to those reported in a study conducted by Fujita et al. No significant prognostic differences were reported in that study; therefore, patients with probable scores should be managed similarly.16
The low presence of patients with definitive scores in the available follow-up data may be attributable to several factors. Prior to their initial consultation, several of these patients were already receiving maintenance treatment, leading to sustained normalized total IgG levels consistent with their clinical response. Additionally, some patients had no baseline total IgG data or had autoantibodies with poor specificity (in 31 of 188 patients with typical or compatible liver biopsy findings, ANA and ASMA were negative). Despite the clear literature highlighting the importance of liver biopsy for diagnostic and prognostic purposes, 43% of patients screened for the simplified AIH score did not undergo it. This was due to a low pretest probability when evaluating the other components of the score (notably 35 patients with typical or compatible biopsies but with normal IgG and negative autoantibodies), clinical contraindications to the procedure, long-standing clinical response despite the absence of biopsy, and administrative difficulties in some cases.
Biopsies are not routinely used for assessing remission and follow-up of fibrosis.37 This highlights the need for non-invasive techniques, such as liver elastography and measuring inflammatory markers, which have emerged as rapid tools. Transient elastography correlates positively with the histological fibrosis stage and has similar accuracy to other chronic liver diseases. However, liver inflammation can confound its interpretation, which may lead to overestimating liver stiffness. This is particularly important to consider in patients receiving immunosuppression. APRI, FIB-4, and AAR scores generally have low diagnostic accuracy.38 Nonetheless, studies have shown that both the immunoglobulin/platelet and lymphocyte/platelet ratios are independently associated with the stage of liver fibrosis in untreated AIH.39
There is considerable debate regarding the diagnosis of AIH, as evidenced by the fact that 63 patients who did not undergo liver biopsy and six patients who did undergo biopsy with typical findings but negative autoantibodies were diagnosed with a probable score for AIH. Seronegative cases present a diagnostic challenge since patients can be diagnosed with AIH using the classic score but result negative with the simplified score, as reported by Sherigar et al.40 This can result in AIH being excluded by the latter method. Furthermore, the low specificity of anti-AIH antibodies can add to the difficulties in diagnosis. In clinical practice, many of these are diagnosed with cryptogenic etiology.
Conclusion
As consistent with previous literature, middle-aged adults and women are the most common demographic for patients with definitive AIH. At the time of diagnosis and in earlier stages, a low percentage of cirrhosis was observed, which may account for the low mortality and absence of acute liver failure, as well as the low incidence of liver transplant requirement. However, more extended follow-up periods are necessary to obtain a more precise estimate of outcomes.
The limited use of liver biopsy represents a significant challenge to the accurate diagnosis of definitive AIH and, therefore, to clinical outcomes. Consequently, proposing non-invasive and more readily available diagnostic alternatives is crucial.











 texto em
texto em