Introduction
In 2020, colorectal cancer was the third most commonly occurring cancer, accounting for 9.5% of new cancer cases, and was also the fourth leading cause of cancer deaths worldwide.1 Approximately 20% to 25% of patients with colorectal cancer have the metastatic liver disease at the time of diagnosis, and an additional 50% develop it in a metachronous scenario.2,3 Among these patients, average survival without treatment is typically less than a year, ranging from 3.8 to 21 months.2
Hepatic metastasectomy, sometimes accompanied by ablation techniques, is the only potential cure for patients, with a survival average of 3.6 years and 5- and 10-year survival rates of 40% and 25%, respectively.4 However, only 10-20% of patients with hepatic damage meet the criteria for surgical resection.5 Nonetheless, the development of new approaches associated with perioperative systemic therapy has increased the number of potentially eligible patients for surgery to 30%.3,6
Several factors have been described to evaluate the prognosis of these patients and determine the benefit of surgical management for metastatic involvement.7 With a better understanding of the tumor biology of colorectal cancer, molecular biomarkers such as KRAS gene mutation have been integrated into prognostic scales. This gene has been extensively studied, and up to 50% of colorectal cancer cases have been reported to have this mutation,3 which is associated with resistance to treatment with monoclonal antibodies against the epidermal growth factor receptor (EGFR). KRAS gene mutation occurs in up to one-third of patients with resectable colorectal cancer hepatic metastases and has a negative prognostic impact due to a higher frequency of extrahepatic metastases, poor response to systemic therapy, and lower overall survival after resection.3,8 Some studies recommend hepatic metastasectomy, whenever possible, in patients without KRAS mutation and suggest evaluating other prognostic factors to decide on the treatment for patients with this mutation.3,9
Surgery remains the most promising option for a potential cure in patients with colon cancer and liver metastases. The liberal use of parenchymal-sparing surgery has increased the possibility of multiple synchronous resections, which can be used in cases of relapse.10,11 However, it is essential to note that parenchymal sparing does not eliminate the need for proper resections to ensure the oncological safety of the procedure. While some studies suggest that narrow margins may yield similar outcomes to the 1-centimeter standard initially described,12,13) it is currently recommended to evaluate factors such as molecular biology, including KRAS status, to determine the optimal margin of resection in these cases.14
This article aims to present the results of a retrospective study conducted at a renowned cancer treatment center in Colombia, focusing on patients who underwent liver resection due to metastases from colorectal cancer, with particular attention to their KRAS gene status. Additionally, the study aimed to assess the effectiveness of anatomical liver resections in patients with mutated KRAS in colorectal cancer.
Materials and methods
This retrospective cohort study includes patients over 18 years old who underwent liver metastasectomy surgery (including anatomic and non-anatomic resections) for colorectal cancer and had a KRAS mutation analysis performed at the National Cancer Institute in Colombia between January 1, 2009, and December 31, 2013. Patients with incomplete follow-up data were excluded to ensure the completeness of the analysis.
The study data were collected by reviewing medical records from the institution and entered into the RedCap program for analysis. Descriptive statistics, including absolute and relative frequencies, measures of central tendency, dispersion, or position, were calculated for qualitative and quantitative variables. The Kaplan-Meier method was utilized to estimate the five-year survival average (both overall and disease-free) in groups using univariate data for patients with mutated and non-mutated (wild-type) KRAS, and the results were analyzed and compared using graphs between patients who underwent anatomic and non-anatomic liver resections using the Logrank test with R-Project software version 3.6.2.
Results
During the study period, 54 patients underwent liver resections for colorectal cancer, but only 35 of them had a study conducted for the KRAS gene (Figure 1). The patients had an average age of 63 (ranging from 42 to 82 years) and a similar distribution between the sexes (57.1% male and 42.9% female). The rectum was the most frequent site of primary tumor location, observed in 40% of patients, followed by the sigmoid colon in 31.4% of cases, while the right colon had a lower frequency of 11.4%. Most patients (85.7%) had a moderate degree of histological differentiation. Among this group, 54.3% presented with stage IV disease, with isolated hepatic metastatic involvement in 84.2% and associated involvement with other organs in 15.8%. Prior to metastasectomy, high levels of carcinoembryonic antigen (CEA) were found in 82% of patients, with a value greater than 5 ng/dL and an average of 7 ng/dL in all patients, with no significant difference between those with mutated and non-mutated KRAS genes (Table 1).
Figure 1 Flowchart of patients with liver resections for colorectal cancer 2009-2013. Source: Authors’ own research.
Table 1 Clinical characteristics of patients who underwent liver resections for colorectal cancer
| Characteristic | Statistics, n (%) |
|---|---|
| Age (years completed) | |
| - Median (min-max) | 63 (42-82) |
| Sex | |
| - Man | 20 (57.14) |
| - Woman | 15 (42.86) |
| Primary tumor | |
| - Right colon | 6 (17.14) |
| - Left colon | 15 (42.86) |
| - Rectum | 14 (40) |
| Neoadjuvant chemotherapy | |
| - No | 23 (65.71) |
| - Yes | 11 (31.43) |
| - No data | 1 (2.86) |
| Synchronous metastasis | |
| - No | 16 (45.71) |
| - Yes | 19 (54.29) |
| Site of metastasis | |
| - Liver | 16 (84.21) |
| - Liver + others | 1 (15.78) |
| Hepatic and primary metastasectomy in a surgical time | |
| - No | 17 (89.47) |
| - Yes | 2 (10.53) |
| Treatment for metastases different from surgery | |
| - No | 16 (94.12) |
| - Yes | 1 (5.88) |
| Degree of differentiation of the primary tumor | |
| - Well differentiated | 4 (11.43) |
| - Poorly differentiated | 1 (2.86) |
| - Moderately differentiated | 30 (85.71) |
| Clinical stage | |
| - II | 5 (14.29) |
| - III | 10 (28.58) |
| - IV | 19 (54.28) |
| - No data | 1 (2.86) |
| Relapses in a place other than the liver | |
| - No | 31 (88.57) |
| - Yes | 4 (11.43) |
| Site other than relapsed liver | |
| - Lung | 4 (100) |
| Neoadjuvant chemotherapy to metastasectomy | |
| - No | 31 (88.57) |
| - Yes | 4 (11.43) |
| Tumor marker before metastasectomy | |
| - Median (min-max) | 7.03 (1.33-88.5) |
| KRAS gene mutation | |
| - No | 20 (57.14) |
| - Yes | 15 (42.86) |
Source: Authors’ own research
KRAS Gene Mutation
Fifteen of the liver resections performed (42.8% of the total) were on patients with a KRAS gene mutation. Of these, 40% had a primary tumor in the left colon (descending or sigmoid), 26.7% in the rectum, and 33.3% in the right colon. In 60% of these patients, metastasis was synchronous. During follow-up, 73.3% of the patients experienced relapse, 19.2% of these relapses occurring exclusively in the liver, and 81.8% associated with another site (lung). The average number of metastatic liver lesions was four, with an average size of 4 cm.
Out of the total of liver resections, twenty patients had wild-type KRAS (57.1%). Of these, 45% had the primary tumor in the left colon, 40% in the rectum, and only 15% in the right colon. Both synchronous and metachronous scenarios were observed in this group in the same proportion. The mean number of metastatic liver lesions was four, and their mean size was 2.7 cm. Among these patients, 70% relapsed, with 64.3% being at the hepatic level and 50% associated with other sites such as the lung, lymph nodes, peritoneum, or bones (Table 2).
Table 2 Clinical characteristics and outcomes of patients with mutated and wild-type KRAS
| Characteristic | Mutated KRAS | Wild-type KRAS |
|---|---|---|
| Age (years completed) | ||
| - Median (min-max) | 66 (45-82) | 59 (42-77) |
| Sex, n (%) | ||
| - Man | 7 (46.67) | 13 (65) |
| - Woman | 8 (53.33) | 7 (35) |
| Primary tumor, n (%) | ||
| - Right colon | 5 (33.33) | 1 (5) |
| - Left colon | 4 (26.67) | 12 (55) |
| - Rectum | 5 (40) | 8 (40) |
| Neoadjuvant chemotherapy, n (%) | 9 (60) | |
| - No | 5 (33.33) | 14 (70) |
| - Yes | 1 (6.67) | 6 (30) |
| Clinical stage, n (%) | ||
| - II | 1 (6.67) | 4 (20) |
| - III | 5 (33.34) | 4 (25) |
| - IV | 9 (60) | 8 (40) |
| - No data | - | 1 (5) |
| Tumor marker prior to metastasectomy | ||
| - Median (min-max) | 6.98 (2.8-42.8) | 8 (1.33-88.5) |
| Number of liver lesions | ||
| - Median (min-max) | 4 (1-12) | 4(1-7) |
| Type of surgery for resection of liver metastases, n (%) | ||
| - Right or left hepatectomy | 1 (6.67) | 2 (10) |
| - Another combined anatomical resection | 2 (13.33) | 3 (15) |
| - Non-anatomic segmental resection | 8 (53.33) | 10 (50) |
| - Anatomical segmentectomy | 4 (26.67) | 5 (25) |
| Measurement of increased liver metastasis (cm) | ||
| - Median (min-max) | 4 (0.7-8) | 2.7 (1-12) |
| Resection status at metastasectomy, n (%) | ||
| - R0 | 12 (80) | 10 (50) |
| - R1 | 3 (20) | 8 (40) |
| - R2 | - | 2 (10) |
| Progression-free survival, months | ||
| - Median (min-max) | 11.5 (0-24.8) | 19.1 (1.9-46.2) |
| State at last contact, n (%) | ||
| - Dead due to illness | 9 (60) | 10 (50) |
| Dead due to another cause | 1 (6.67) | 1 (5) |
| - Alive with disease | 4 (26.67) | 6 (30) |
| - Alive without disease | 1 (6.67) | 3 (15) |
| Overall survival, months | ||
| - Median (min-max) | 34.1 (0.5-82.6) | 46.4 (2.7-152.4) |
Source: Authors’ own research
Chemotherapy and Radiation Therapy
Regarding the other types of therapies received, 31.43% of patients underwent neoadjuvant chemotherapy for the primary tumor, with a majority of patients (60%) receiving the 5-fluorouracil plus leucovorin combination, which was common among rectal tumors in this study. Additionally, 85.2% of patients received adjuvant therapy following resection of the primary tumor, with the majority (63.3%) receiving regimens based on 5-fluorouracil, leucovorin, and oxaliplatin. Only 16% of patients received targeted therapy, with 75% receiving anti-VGFR therapy and 25% receiving anti-EGFR therapy. Among the 45.7% of patients in the metachronous scenario, 11.4% received additional chemotherapy regimens before undergoing liver resection surgery, primarily based on 5-fluorouracil and irinotecan. However, all second hepatic relapse cases received systemic therapy prior to resection.
In the analysis of patients based on their KRAS status, 33.3% of patients with mutated KRAS and 30% of patients with wild-type KRAS received chemotherapy before undergoing liver resection. However, 85% of patients with mutated KRAS and 87% with wild-type KRAS received adjuvant systemic therapy. Furthermore, 28.6% of patients received radiotherapy for the locoregional management of primary rectal tumors.
Hepatic Resection
Among the patients in the synchronous scenario, only 10.5% underwent simultaneous liver resection with the primary tumor surgery. Non-anatomic segmental resections were performed in 54.3% (n=19) of patients. Anatomic resections were performed in 45.7% of the patients, with segmentectomy being the most frequent type of anatomic resection in 25.7% of cases, followed by combined resections (which included anatomic resection of several segments or non-anatomic resection) in 14.3%, and right or left hepatectomy in 5.7% and 2.9% of cases, respectively.
The study reported that R0 resection was achieved in 62.9% of patients, while 31.4% achieved R1 resection. In addition, two patients received surgical resection along with other local therapies for residual lesions. It is also worth noting that all patients who underwent a second liver resection were classified as R0.
Of the patients with mutated KRAS, anatomical resections were performed in 46.6% (n=16), with 13.3% undergoing combined resections. R0 resection was achieved in 80% of this group. For patients with wild-type KRAS, anatomic resections were performed in 50% of cases, with segmentectomies and combined resections being the most common types. In this group, 50% of resections were considered R0, 40% R1, and only 10% were considered R2 (Table 3).
Table 3 Characteristics of liver resections in all patients
| Characteristics of liver resections | n (%) |
|---|---|
| Number of liver lesions | |
| - Median (min-max) | 4.5 (1-12) |
| Surgery for resection of liver metastases | |
| - Right or left hepatectomy | 3 (8.57) |
| - Other combined anatomic resections | 5 (14.29) |
| - Non-anatomic segmental resection | 18 (51.43) |
| - Anatomical segmentectomy | 9 (25.71) |
| Measurement of increased liver metastasis (cm) | |
| - Median (min-max) | 3 (0.7- 12) |
| Resection status at metastasectomy | |
| - R0 | 22 (62.86) |
| - R1 | 11 (31.43) |
| - R2 | 2 (5.71) |
| Chemotherapy adjuvant to metastasectomy | |
| - No | 4 (11.43) |
| - Yes | 29 (82.86) |
| - No data | 2 (5.71) |
| Nonsurgical management of liver metastases, n (%) | |
| - Other | 4 (11.4) |
| - Radioablation | 2 (5.7) |
Source: Authors’ own research.
Outcomes
The median follow-up duration for the study was 39 months. Unfortunately, 54.3% of the patients died from their oncological disease, while 5.7% died from other causes. At the end of the study, only 40% of the patients survived, and of these, only 28.5% were deemed disease-free and not receiving any active treatment (Table 4).
Table 4 Outcomes of patients who underwent liver resections for colorectal cancer
| General patient outcomes | Statistics |
|---|---|
| Progression-free survival (months) | |
| - Median (min-max) | 15.6 (0-46.2) |
| State at last contact, n (%) | |
| - Dead due to illness | 19 (54.29) |
| - Dead from a different cause | 2 (5.71) |
| - Alive with disease | 10 (28.57) |
| - Alive without disease | 4 (11.43) |
| Overall survival (months) | |
| - Median (min-max) | 37.1 (0.5-152.4) |
Source: Authors’ own research.
In the subgroup analysis based on KRAS gene status, it was observed that among patients with KRAS mutation, 60% of them died due to cancer-related causes, 6.7% died from causes unrelated to cancer, and only 33% of patients were alive at the end of the study follow-up, out of which 80% had active disease. Conversely, in patients with wild-type KRAS, 50% had died due to cancer-related causes at the end of the study, and 66% had active disease among the living patients.
The study showed that the overall survival had a median of 37.1 months. When the analysis was based on the KRAS gene status, patients with mutated KRAS had a median survival of 34.2 months, while those with wild-type KRAS had a more prolonged median survival of 46.5 months. In the subgroup analysis based on the type of resection performed, patients with mutated KRAS who underwent anatomic resections had a longer median survival of 43.5 months compared to those who underwent non-anatomic resections with a median survival of 23.5 months. For patients with wild-type KRAS, the median survival was 34.5 months for non-anatomic resections and 41.3 months for anatomic resections.
Discussion
Metastatic colorectal cancer is a complex condition characterized by significant biological variability. Therefore, it is essential to examine each case individually when considering locoregional and systemic management options.15 With regards to liver metastases from colorectal cancer, various studies have investigated multiple clinical and pathological factors that can influence the prognosis and benefits of liver resection surgery. Some preoperative scoring systems have also been analyzed to support the selection of suitable candidates for hepatic resection.7,16
In recent decades, KRAS and NRAS gene mutations have been analyzed as biological markers. Studies have shown that these mutations are associated with resistance to monoclonal antibody therapy against EGFR in cases of metastatic disease.17,18 Furthermore, these mutations are linked to a lower response rate to conventional management, faster disease progression, and poorer survival outcomes.19
The activation of the EGFR-activating-dependent RAS/RAF signaling pathway through receptor binding is caused by an oncogenic mutation in KRAS, leading to constant stimulation of proliferation, angiogenesis, resistance to apoptosis, and increased metastatic capacity.20,21 Consequently, it can be inferred that EGFR inhibitors, which operate at a higher level than the activation of the KRAS pathway, are ineffective in patients with a mutation that sustains the active pathway.
Several studies have investigated the impact of KRAS gene status on the outcomes of patients with liver metastases due to colorectal cancer who undergo liver resection. However, the findings have not been consistent across studies, with some reporting adverse effects for patients with mutated KRAS while others not.22-25 A meta-analysis conducted by Passiglia concluded that patients with mutated KRAS had worse outcomes in terms of recurrence and survival.3
Recently, some researchers have reported specific outcomes for patients with mutated KRAS when offered better local surgical control with anatomic resections. This alternative has shown promise for improving survival rates compared to systemic therapies alone without local control of metastatic involvement. However, the results of studies on this topic are contradictory (Table 5).26-28
Table 5 Studies reporting oncological outcomes according to the type of liver resection in relation to KRAS gene status in patients with liver metastases due to colorectal cancer
| Study | Margonis et al. | Choi M. et al. | Kwai T. et al. | Acevedo et al. |
| Number of patients | 389 | 250 | 290 | 35 |
| Year | 2017 | 2022 | 2022 | 2022 |
| Oncology outcomes | DSF Anatomical KRASmut: 33.8 months Non-anatomical: 10.5 months | DSF Anatomical KRASmut: 11 months Non-anatomical: 9 months | 5-year OS KRASmut Anatomical: 55% Non-anatomical: 53% KRASwt Anatomical: 81% Non-anatomical: 58% | Average OS Anatomical KRASmut: 43.5 months Non-anatomical: 23.5 months KRASwt Anatomical: 34.5 months Non-anatomical: 41.3 months |
| Comments | Non-anatomic resections are associated with worse DFS in patients with KRAS mutation tumors. | The presence or absence of the KRAS mutation did not show a significant association with DFS, regardless of the type of resection, and was not considered a significant prognostic factor. | Anatomic resection was an independent prognostic factor for DFS and OS in KRASwt patients. In contrast, anatomic resection was not associated with SLE or OS in KRAS mutation patients. | Anatomic resection was a factor associated with improved survival in patients with mutated KRAS. |
KRASmut: KRAS gene mutation; KRASwt: wild state or no mutation of the KRAS gene; OS: overall survival; DFS: disease-free survival. Source: Authors’ own research
In our case series, 46.6% of patients with KRAS mutation and 50% with wild-type KRAS underwent anatomic resections, which were planned based on the number and location of metastatic lesions without considering the mutation status for the type of resection at the moment. We observed that in the group with mutated KRAS, anatomic resections resulted in an 85% higher average survival rate (43.5 vs. 23.5 months) compared to non-anatomic resections (Figure 2).
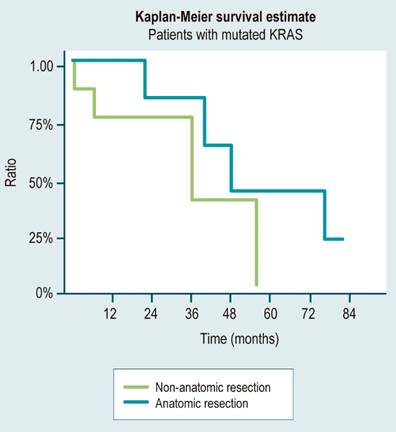
Figure 2 Overall survival of patients with KRAS mutation according to the type of resection. Source: Authors’ own research.
Several studies have demonstrated that patients with liver metastases due to colorectal cancer benefit more from surgery than from systemic management, with improved survival rates.29-31 This benefit also extends to patients with mutated KRAS, who have a worse prognosis. In fact, studies have reported an average survival of 34 to 40 months for patients with mutated KRAS who underwent surgery, compared to only 10.6 months for those who received systemic management with chemotherapy alone.32-34 In our study, patients with mutated KRAS who underwent anatomic resections had an average survival of 43.5 months, which was similar to the average survival of 41.5 months for those with wild-type KRAS who underwent anatomic resections. However, patients with mutated KRAS who underwent non-anatomic resections had a markedly lower average survival of 23.5 months, compared to 34.5 months for those with wild-type KRAS who underwent non-anatomic resections.
There is a limited number of studies that describe or recommend anatomic resections, particularly in patients with mutated KRAS status.26-28 These studies have presented varying results regarding overall survival and disease-free survival. However, due to the higher frequency of micrometastases and R1 resections in this patient population,34,35 achieving local control with parenchymal-sparing liver resections may be more challenging.
This paper has limitations as it is a retrospective study with relatively few patients. However, the results are consistent with those reported by other authors, showing better survival outcomes for patients with mutated KRAS who undergo greater margin or anatomic liver resections. Although there is still limited evidence, the results of this retrospective series are valuable and encourage further research to enable appropriate decision-making for patients with mutated KRAS who are candidates for liver resections, especially for those with unfavorable prognoses in different oncological outcomes.











 texto en
texto en 


