Introduction
Whipple’s disease (WD) is a chronic systemic disease likely to manifest in the digestive system, particularly in the small intestine. It was first reported in 1907 by George H. Whipple, who named it intestinal lipodystrophy.1,2 The disease is caused by a gram-positive bacterium, Tropheryma whipplei (TW), which belongs to the Actinomycetaceae family, and causes an infection with nonspecific symptoms. However, the typical symptoms usually start with arthralgia and then diarrhea, weight loss, fever, adenopathy, and, in some cases, neurological, cardiac, or ocular manifestations. Although atypical, the disease may also present as a febrile syndrome of unknown origin.1-4
This study aims to provide a systematic literature review that characterizes patients with WD.
Materials and methods
Type of Study
The systematic literature search for this study was carried out in accordance with the 2020 “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) statement and the Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.022.
Population
The study included all individuals who had been diagnosed with WD and had received treatment, as well as articles published from 2015 to 2021. The latest developments related to clinical presentations, diagnosis, laboratory tests, treatment, and prognosis were also taken into consideration.
Inclusion Criteria
We included case reports and case series articles published in English or Spanish within the last six years.
Variables
The variables analyzed in this study were sex (male/female), country of origin, year of publication, medical history, clinical manifestations, laboratory tests, and medical management approaches used.
Search Strategies
Between September 1 and September 15, 2021, the researchers conducted a search using the following databases: PubMed/Medline, Scopus, Scielo, Science Direct, Embase, Cochrane Library, BIREME, Proquest, and Redalyc. The search terms used were “Whipple Disease” (Enfermedad de Whipple) or “Tropheryma”, based on the DeCS [MeSH] system.
Search Restrictions
The search was restricted to literature related to humans and published in English and Spanish.
Data Extraction
The articles found in the literature search were recorded in an MS Excel database, and duplicates were removed. We discussed doubts and reasons for possible exclusions as a team and made consensus-based decisions. The statistical software SPSS version 22 was used to analyze the data. Descriptive statistics were used to conduct a univariate analysis and determine the absolute and relative frequencies of the qualitative variables, as well as measures of central tendency and dispersion for the quantitative variables. Finally, we reviewed the full articles to select those to be included in this study.
Biases
In this study design, it is important to control several biases. The first is related to poor population selection, which is why we created inclusion and exclusion criteria. The second one is measurement bias, which we addressed using a data collection sheet to be applied by the researchers. Finally, we had to deal with potential information gaps since the data come from case reports and case series records that may have incomplete information.
Results
Selection of Studies
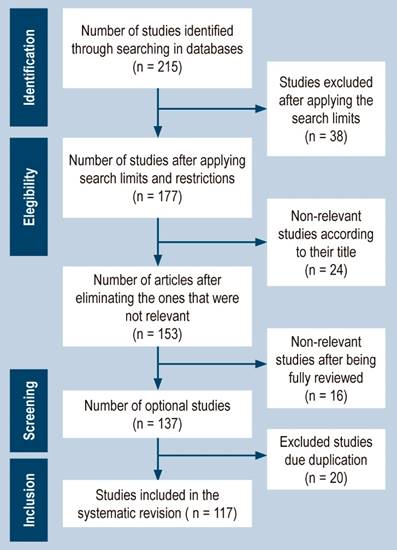
After implementing the search strategy as described, we initially identified 215 articles from the selected databases. However, we excluded 38 articles due to not meeting our search criteria. Following this, we screened the titles and abstracts of the remaining 177 articles and eliminated 24 articles. This resulted in 153 articles that were eligible for full-text review. After reviewing the full-text articles, we excluded 16 articles that did not meet the study’s inclusion criteria, resulting in 137 articles. We then removed 20 duplicate articles, resulting in 117 published articles consisting of case reports and case series. We adhered to the PRISMA statement guidelines for the selection process of the included studies, and a flowchart outlining the study selection process can be found in Figure 1.
Sociodemographic Characteristics of Patients
The data interpretation regarding the most common age of onset for WD reveals that it can occur at any stage of life, with documented cases ranging from a 4-year-old girl to an 82-year-old man. However, the disease’s highest prevalence falls within the approximate age range of 50 to 65 years, with 67 reported cases. Furthermore, a greater incidence was observed in the 50-58 (2.9%) and 63 (6.6%) age groups. Males showed a higher incidence rate, with 96 cases and a percentage of 70.6% out of the total cases.1-117
Clinical Characteristics
The researchers conducted an analysis of the clinical case series and identified the most common manifestations of WD. Arthralgia was found in 83 patients (61%), weight loss in 64 (47.1%), diarrhea in 59 (43.4%), fever in 45 (33.1%), abdominal pain in 31 (22.8%), and neurological symptoms in 30 cases (22.1%), as shown in Table 1.1-117
Table 1 Clinical characteristics
| Prevalence | n | % | Prevalence | n | % |
|---|---|---|---|---|---|
| Clinical manifestations | Studies | ||||
| Diarrhea | 59 | 43.4 | Hemoglobin (anemia) | 40 | 29.4 |
| Bloating | 14 | 10.3 | Leukocytosis | 21 | 15.4 |
| Rectal bleeding | 3 | 2.2 | Neutrophilia | 11 | 8.1 |
| Abdominal pain | 31 | 22.8 | Increased ESR | 114 | 83.8 |
| Dyspnea | 19 | 14.4 | PCR for T. Whipple positive | 86 | 63.2 |
| Headache | 4 | 2.9 | Human rheumatoid factor | 1 | 0.7 |
| Fever | 45 | 33.1 | Anatomopathological examination with PAS granules | 65 | 47.8 |
| Hyporexia | 11 | 8.1 | Fecal occult blood | 3 | 2.2 |
| Anorexia | 7 | 5.1 | Imaging findings | 90 | 66.2 |
| Weight loss | 64 | 47.1 | Colonoscopy | 9 | 6.6 |
| Asthenia | 10 | 7.4 | Endoscopic findings | 25 | 18.4 |
| Polyarthritis | 25 | 18.4 | Biopsy findings | 69 | 50.7 |
| Myalgias | 7 | 5.1 | Autopsy | 1 | 0.7 |
| Arthralgias | 83 | 61.0 | |||
| Adynamia | 3 | 2.2 | |||
| Fatigue | 14 | 10.3 | |||
| Weakness | 10 | 7.4 | |||
| Vomit | 8 | 5.9 | |||
| Neurological symptoms | 30 | 22.1 | |||
| Ophthalmoplegia | 3 | 2.2 | |||
| Cardiac symptoms | 10 | 7.4 | |||
| Tachycardia | 3 | 2.2 | |||
| Hyperpigmentation of the skin | 9 | 6.6 | |||
| Apathy | 2 | 1.5 | |||
| Irritability | 2 | 1.5 | |||
| Lymphadenopathy | 24 | 17.6 | |||
| Pericarditis | 1 | 0.7 | |||
| Endocarditis | 2 | 1.5 | |||
| Pleural effusion | 3 | 2.2 | |||
| Personality changes | 4 | 2.9 | |||
| Uveitis | 5 | 3.7 | |||
| Insomnia | 2 | 1.5 | |||
| Night sweats | 12 | 8.8 | |||
| Hypotension | 4 | 2.9 | |||
| Ocular symptoms | 18 | 13.2 | |||
| Depression and anxiety | 5 | 3.7 | |||
| Edema in lower limbs | 18 | 13.2 | |||
| Swelling of limbs | 1 | 0.7 | |||
| Paresthesia in lower limbs | 3 | 2.2 | |||
| Hypoesthesia and hyporeflexia | 2 | 1.5 |
PAS: Periodic acid-Schiff. Source: Authors’ own research.
Based on the clinical cases analyzed by the research group, it was found that the three most commonly used diagnostic tools for generating a possible diagnosis were PCR for T. Whipple in 86 patients (63.2%), biopsy in 69 patients (50.7%), and pathological examination with positive staining for Periodic acid-Schiff (PAS) in 65 cases (47.8%). In addition, imaging findings from tests such as radiography, magnetic resonance imaging (MRI), and computed tomography (CT) were essential for the diagnosis, as they were helpful in 90 patients (66.2%). The erythrocyte sedimentation rate (ESR), high in 114 patients (83.8%), was also significant in aiding diagnosis.
Therapeutic characteristics
The results of the study showed that antibiotic therapy was the preferred approach for treating WD, with a high tendency towards the use of trimethoprim/sulfamethoxazole (61.0%), ceftriaxone (57.4%), and doxycycline (31.6%). Hydroxychloroquine (26.5%) and corticosteroids/prednisone (15.4%) were also used. Other antibiotic-based treatments, such as cefotaxime, meropenem, vancomycin, imipenem, tigecycline, and cefixime, were used less frequently. A detailed description of the results can be found in Table 2.1-117
Table 2 Therapeutic features1-117
| Prevalence | n | % |
|---|---|---|
| Therapeutic characteristics | ||
| Trimethoprim/sulfamethoxazole | 83 | 61 |
| Ceftriaxone | 78 | 57.4 |
| Metronidazole | 1 | 0.7 |
| Vancomycin | 3 | 2.2 |
| Penicillin | 6 | 4.4 |
| Meropenem | 7 | 5.1 |
| Tigecycline | 2 | 1.5 |
| Doxycycline | 43 | 31.6 |
| Cefotaxime | 1 | 0.7 |
| Cefixime | 2 | 1.5 |
| Imipenem | 3 | 2.2 |
| Ciprofloxacin | 1 | 0.7 |
| Hydroxychloroquine | 36 | 26.5 |
| Corticosteroids or prednisone | 21 | 15.4 |
Source: Authors’ own research.
In addition, other treatment options were employed to manage specific associated symptoms. These included methotrexate (1.5%), non-specified antibiotics (1.5%), nonsteroidal anti-inflammatory drugs (NSAIDs) (1.5%), ofloxacin/prednisone drops (0.7%), palliative stereotactic surgery (0.7%), caspofungin (0.7%), and gentamicin plus noradrenaline plus dopamine (0.7%), as reported in Table 3.
Table 3 Other treatment options
| Prevalence | n | % |
|---|---|---|
| Therapeutic characteristics | ||
| NSAIDs | 2 | 1.5 |
| Amoxicillin | 1 | 0.7 |
| Gentamicin + Amoxicillin | 1 | 0.7 |
| Caspofungin | 1 | 0.7 |
| Ceftazidime | 1 | 0.7 |
| Cyclophosphamide | 1 | 0.7 |
| Co-trimoxazole | 4 | 2.9 |
| Streptomycin | 1 | 0.7 |
| Gentamicin + noradrenaline + dopamine | 1 | 0.7 |
| Leflunomide | 1 | 0.7 |
| Methotrexate | 2 | 1.5 |
| Ofloxacin drops + prednisone drops | 1 | 0.7 |
| Piperazine | 1 | 0.7 |
| Sulfadiazine | 1 | 0.7 |
| Non-specified antibiotic | 2 | 1.5 |
| Stereotactic palliative surgery | 1 | 0.7 |
Source: Authors’ own research.
Discussion
WD is a systemic condition caused by Tropheryma whipplei, a gram-positive bacterium. The disease is named after the Greek words trophe (food) and eryma (barrier) due to its association with poor absorption of nutrients. It was first described in 1907 by George Hoyt Whipple as a chronic and systemic disease linked to intestinal lipodystrophy. It was not until 1952 that its etiology was postulated to be bacterial, based on clinical improvement after antibiotic treatment. Electron microscopy findings in 1960 provided further evidence for this hypothesis, and its ribonucleic acid (RNA) was amplified using PCR in 1990. Cultivation of the bacterium was achieved in 1997.42 The disease can occur at any age, as there are reports of cases from individuals aged 4 to 82.1-117 While the highest prevalence is found among individuals between 50 to 65 years old, the disease onset can occur at any age. Males have a higher predisposition to the disease, with 96 cases reported and a percentage of 70.6.1-117
The clinical presentation of WD is variable and not specific, with different organs affected and requiring diverse treatments. The disease may manifest as gastrointestinal histological lesions or isolated neurological cases. Typically, joint involvement is the first clinical sign, as observed in 83 patients (61%), consistent with a study by Moreno García (60% of cases). Weight loss is present in 64 patients (47.1%), diarrhea in 59 (43.4%), fever in 45 (33.1%), abdominal pain in 31 (22.8%), and neurological symptoms in 30 cases (22.1%). Joint involvement typically appears as intermittent migratory arthralgia, arthritis, or both, without deformity. It usually affects multiple joints, although it can be oligoarticular in large joints.9
Ocular involvement is not common in WD and typically presents at a later stage. It should be considered when bilateral choroidal granulomatosis is observed in the context of an atypical systemic disease. A limited number of cases of ocular involvement in WD have been reported in recent scientific literature, with eighteen cases identified in the review, representing a prevalence of 13.2%.29
According to Gundling F et al., central nervous system (CNS) manifestations occur in up to 15% of cases. These may occur rarely with little or no gastrointestinal damage, as isolated involvement, or associated with gastrointestinal disease. They may also occur as neurological relapse in previously treated disease,58 which is consistent with reports of cases that found CNS involvement in 30 patients (22.1%). Histological alterations in the brain have been found in autopsies. This review found alterations in one case (0.7%) where WD organisms significantly affected several organ systems, confirmed by histochemical and molecular evaluation.
The frequency of cardiac involvement in WD varies widely among different studies. Our research found involvement in ten cases (7.4%), while other authors, such as Parodi R., reported a range of 17% to 55% of cases. Pericarditis is a common manifestation, while myocarditis is less frequent but can be the initial presentation of cardiac failure or sudden death. Endocarditis can also occur but is difficult to diagnose due to negative blood cultures, lack of fever, and valve destruction. Its diagnosis is confirmed through PCR analysis of surgically obtained valvular material.9
In terms of diagnosing Wilson’s disease (WD), the three most commonly utilized diagnostic tools are the PCR for TW, biopsy, and anatomopathological examination with PAS granules. Fernández-Mondelo et al. propose two diagnostic criteria for WD: positive PAS staining in a small intestine biopsy and positive results in two tests (PAS, PCR, or immunohistochemistry) of TW from gastrointestinal or extraintestinal samples. Gastroscopy with biopsy remains the most frequently used technique for diagnosing WD. Macroscopically, a woolly, yellow, and pale mucosa alternating with an erythematous, erosive, or slightly friable mucosa in the post-bulbar region of the duodenum or jejunum is a characteristic finding. Biopsies taken at this level reveal positive foamy macrophages for PAS. Identifying the bacillus through PCR in the same duodenal tissue can confirm the diagnosis. In patients with suspected WD but without gastrointestinal symptoms, samples should be collected from relevant anatomical sites such as synovial fluid and lymph nodes, among others.91
Differential Diagnoses
When joint symptoms are predominant, other rheumatic diseases should be ruled out before considering WD. In cases where joint disease does not improve or worsens with biological therapy, WD should be suspected. HIV infection can also present with similar symptoms, including enteropathy and wasting syndrome. If endocarditis is present, the most common pathogens should be ruled out first. The presence of systemic granulomas can be mistaken for sarcoidosis. In cases of abdominal involvement, other causes of malabsorptive syndrome should be investigated, such as celiac disease and other infections. A diagnosis of abdominal angina9 should also be considered for patients with vascular disease.
The recommended treatment for WD includes antibiotics that can penetrate the blood-brain barrier, such as trimethoprim/sulfamethoxazole or ceftriaxone, to prevent neurological relapses.8 In our research, we observed a preference for trimethoprim/sulfamethoxazole (83 cases; 61.0%) followed by ceftriaxone (78 cases; 57.4%). Currently, the recommended treatment involves ceftriaxone (2 g/day) or meropenem (3 g/day) administered parenterally, followed by trimethoprim/sulfamethoxazole (800-160 mg/ times daily) administered orally for at least one to two years. In cases of isolated neurological forms, ceftriaxone (2-4 g/day) is administered intravenously for 15-30 days, followed by trimethoprim/sulfamethoxazole (1600-320 mg/day) or doxycycline (200 mg/day) or cefixime (400 mg/day) until cerebrospinal fluid (CSF) PCR results are negative.8
The treatment goals for Whipple’s disease are to minimize morbidity, prevent complications, and eliminate the infection. Without appropriate treatment, the mortality rate is almost 100%. However, the prognosis is favorable with complete antibiotic therapy.











 text in
text in