Clinical profile
Most patients with nonalcoholic fatty liver disease (NAFLD) are typically asymptomatic, a common characteristic of liver diseases. Patients may sometimes report symptoms such as asthenia, adynamia, or pain in the right hypochondrium. As the disease progresses to advanced stages, signs and symptoms of portal hypertension or cirrhosis may manifest.1 NAFLD is commonly seen in overweight or obese individuals, although it may also affect those with a lower body mass index.1,2 Hepatomegaly due to fatty liver infiltration has been reported in 5% of patients with fatty liver and 18% of those with nonalcoholic steatohepatitis (NASH).3-5 This condition may lead to abdominal pain, although other potential causes should always be considered.1
Laboratory
Elevated aminotransferases, typically two to five times the normal value, often prompt patients to seek initial medical consultation. However, this elevation cannot predict the extent of liver inflammation or fibrosis. In addition, normal levels of alanine aminotransferase (ALT) do not rule out a diagnosis of fatty liver or significant histological damage.6-8
Other laboratory findings in patients with nonalcoholic fatty liver disease (NAFLD) include an elevation of alkaline phosphatase (AP) by two or three times the normal value, an increase in serum ferritin concentration, or elevated transferrin saturation.9 A ferritin level greater than 1.5 times the normal value in patients with fatty liver is associated with advanced liver fibrosis.10 Moreover, albumin, bilirubin, and coagulation times are only altered in advanced stages of cirrhosis.9
Radiology
Any imaging modality can show fatty liver. The most commonly used test is abdominal or hepatic ultrasound, and steatosis manifests as a diffuse increase in echogenicity of the liver parenchyma or a bright liver.11 A meta-analysis using liver biopsy as the gold standard reported a sensitivity (S) and specificity (E) of 85% and 94%, respectively.12 However, in obese patients and those with less than 30% fat content, these values are lower, with reported sensitivities ranging from 49% to 66% and specificities from 77% to 93.1%.11,13
Computed tomography (CT) can diagnose hepatic steatosis with a sensitivity of 82% and a specificity of 100% when the fat content is equal to or greater than 30%.14 However, with lower fat contents, these values are reduced to 50% and 83%, respectively.11 CT is a rapid method that does not depend on the operator, but it is important to consider the radiation exposure to which patients are subjected.
When nuclear magnetic resonance (NMR) is used in studies where liver biopsy is considered the gold standard test for detecting steatosis, sensitivity (S) has been found to range between 88% and 95% and specificity (E) between 63% and 98%, respectively.15,16 However, when the detection of histological steatosis is reduced to ≥ 5%, reported S values range between 76.7% and 90.0%, and E values between 87.1% and 91%.
The proton density fat fraction (PDFF) estimated by magnetic resonance spectroscopy (MRS) is an accurate and reproducible non-invasive biomarker for hepatic steatosis.17,18 However, spectroscopic sequences are not available on all scanners, and the technique is not routinely used due to its cost.
Liver biopsy
Liver biopsy remains the preferred method for diagnosing fatty liver disease and accurately distinguishing between simple steatosis, steatohepatitis, and cirrhosis, which has prognostic implications and guides patient management, often motivating them to make beneficial lifestyle changes.19-25 It is recommended in cases of:
Patients at high risk of fibrosis or cirrhosis, such as those with obesity, diabetes, dyslipidemia, or serum ferritin levels greater than 1.5 times the upper limit of normal, when non-invasive tests cannot rule out advanced fibrosis.
Patients with suspected but unconfirmed fatty liver after initial laboratory and imaging studies.
Suspected advanced liver disease associated with fatty liver, peripheral signs of chronicity or cirrhosis, splenomegaly, and cytopenia.
Need for determining the severity of the disease or excluding other phenomena.
Findings
To diagnose fatty liver through histology, a liver tissue sample must show the presence of 5% or more hepatocytes with steatosis. The severity of the condition can be classified as mild (5%-33%), moderate (34%-66%), or severe (>66%), based on the percentage of hepatocytes with steatosis present in the sample.21,22
Distinguishing simple steatosis from NASH requires a careful examination of the histological findings. Simple steatosis may exhibit lobular or portal inflammation with hepatocyte ballooning or hepatocyte ballooning without inflammation.20,23 In contrast, NASH is characterized by the presence of hepatic steatosis combined with hepatocyte ballooning and hepatic lobular inflammation, typically observed in the acinar zone 3.20,23 While fibrosis is not a necessary diagnostic feature, it may be present. As fibrosis progresses to cirrhosis, steatosis and inflammation may disappear, resulting in the diagnosis of “cryptogenic” cirrhosis.23
Several studies have demonstrated that histological parameters such as hepatocellular ballooning and inflammation, in addition to the age of patients, are the best predictors of fibrosis progression in fatty liver disease.26 Other studies have shown that the presence of fibrosis in the initial biopsy or its progression in subsequent biopsies is strongly associated with adverse outcomes and increased mortality in fatty liver disease.27,28 Therefore, assessing the presence of fibrosis in patients with fatty liver disease is crucial.
Non-invasive determination of liver fibrosis
Non-invasive tests provide an excellent alternative to liver biopsy for determining the degree of liver fibrosis and establishing the stage of fibrogenesis (F0-F4) in any patient. A score ≥ F2 and advanced fibrosis ≥ F319,20,24,25,29 are considered significant fibrosis. Two categories of non-invasive liver fibrosis tests are available: serological and image-based. Combining them is the current trend, using whichever is locally available, resulting in fewer patients with an indeterminate fibrosis score and higher specificity.25,29,30
Serological Tests
Several serum marker products have been validated for the diagnosis of liver fibrosis, including:
APRI
FIB-4
User ratings for NAFLD fibrosis
BARD Score
FibroTest/FibroSURE
Hepascore
FIBROSpect
ELF score (panel of the European Hepatic Fibrosis Study Group)
While non-invasive tests can differentiate between patients with significant fibrosis (F2 to F4) and those without (F0 to F1), they are not as reliable in distinguishing between multiple stages of fibrosis, leading to indeterminate results in up to 65% of cases.25,29,30 Some markers are still helpful in the field and include the following.
APRI or relationship between AST and platelets
The usefulness of APRI has been studied in patients with various diseases, including hepatitis C virus (HCV), human immunodeficiency virus (HIV), HIV-HCV coinfection, and alcoholic liver disease.29 A meta-analysis of 40 studies found that an APRI cutoff point of 0.7 had a sensitivity of 77% and a specificity of 72% in predicting significant fibrosis (F2 to F4), and an APRI cutoff point of 1.0 had a sensitivity of 76% and a specificity of 72% in predicting cirrhosis (F4).31 In patients with NAFLD, the ability of APRI to predict adverse liver-related outcomes was examined in a retrospective series of 320 patients,32 and the area under the ROC curve (AUC) to predict these outcomes was 0.80. The AUC to predict liver death or transplantation was 0.63.
The FIB-4
The FIB-4 test combines platelet count, ALT, AST, and age and has typically been studied in the context of hepatitis C. It is also useful in predicting advanced fibrosis in fatty liver disease.33 A study found that the area under the ROC curve (AUC) to predict adverse outcomes using FIB-4 was 0.81, and to predict death or liver transplantation, it was 0.67.32 The test is interpreted using two diagnostic thresholds: a lower threshold of <1.30 to exclude advanced fibrosis and an upper threshold of >2.67 to confirm it.29
User Ratings for NAFLD Fibrosis
The NAFLD fibrosis score is calculated according to patient-age-based routine laboratory tests, including BMI, blood glucose levels, aminotransferase levels, platelet count, and albumin.34 In a validation study, a high cutoff value (>0.676) was associated with an 82% positive predictive value for advanced fibrosis (F3 to F4) with a sensitivity of 43% and specificity of 96%, while a low cutoff value (< -1.455) was associated with an 88% negative predictive value with a sensitivity of 77% and specificity of 71%.33
BARD Score
The BARD score takes into account BMI, AST/ALT ratio, and the presence of diabetes mellitus.35 A study of 126 patients with fatty liver found positive and negative predictive values for advanced fibrosis of 69% and 96%, respectively, with an AUC of 0.87.36 Another study reported AUC values of 0.73 and 0.66 for predicting adverse outcomes related to the liver and liver death or transplantation, respectively.32
Image-Based Testing
Liver stiffness is determined using mechanical waves in a process called elastography, which measures the propagation speed through tissue. The most common type of elastography is ultrasound-based and includes FibroScan® (or transient elastography), real-time 2D shear wave elastography (2D-SWE) called SuperSonic®, acoustic radiation force impulse (ARFI) elastography, and magnetic resonance elastography (MRE). 2D-SWE and MRE combine elastography with conventional liver imaging in a single session.37 While ultrasound-based tests are excellent at predicting healthy liver, advanced fibrosis, or cirrhosis, their accuracy in the intermediate stages should be interpreted with caution.25,29,38
Transient Elastography or FibroScan®
FibroScan® is the most extensively studied device for measuring liver stiffness. It utilizes two probes: the classic M and the XL, which were developed to optimize the measurement and reduce the failure rate in obese patients.29,38,39 The two diagnostic thresholds with FibroScan® to exclude or suspect advanced hepatic fibrosis (≥F3) are < 7.9 kPa and > 9.6 kPa, respectively, with a negative predictive value of 96% and a sensitivity of 89%.40 The indeterminate gray zone between the two thresholds accounts for 10%-15% of patients. Two-thirds of patients with a result > 9.6 kPa have advanced liver fibrosis, corresponding to a positive predictive value of 67%.40 Although it can achieve a diagnostic accuracy (AUC) greater than 0.92 for advanced fibrosis, it is less precise in patients with fatty liver in intermediate stages.41
Acoustic Radiation Force Impulse Imaging
ARFI uses a high-intensity and short-duration acoustic pulse to measure tissue displacement in the same direction.42 The diagnostic ability of ARFI and TE for detecting hepatic fibrosis may be similar. In a study, the AUC for ARFI versus TE to diagnose fibrosis stage ≥ F2 was 0.77 and 0.74, respectively. For diagnosing fibrosis ≥ F4, the AUC for ARFI versus TE was 0.84 and 0.80, respectively. However, in patients without obesity, ARFI performed slightly better in diagnosing stage ≥ F4 fibrosis with an AUC of 0.92, a difference not observed with TE.43
2D Shear Wave Elastography, 2D-SWE, or SuperSonic®
SuperSonic® is an elastography technique that offers simultaneous real-time grayscale images of the tissue being studied. This technique is integrated into conventional ultrasound scanners, allowing both procedures to be performed in the same session.37,38,44) A prospective controlled study on patients with fatty liver showed that SuperSonic® had an AUC of 0.84 for diagnosing stage ≥ F2 fibrosis, 0.88 for stage ≥ F3 fibrosis, and 0.93 for cirrhosis.45 Other studies have reported similar results in diagnosing significant advanced fibrosis and cirrhosis.46,47
Magnetic Resonance Elastography
MRE, unlike ultrasound-based elastography, enables the examination of the entire liver and is not limited to a specific target for sampling. It is conducted using a standard MRI scanner equipped with extra hardware and software, and elastography and morphological imaging can be performed simultaneously. A meta-analysis revealed that the sensitivity and specificity for detecting fibrosis stage ≥ F2 were 79% and 81%, respectively; for fibrosis ≥ F3, they were 85% and 85%, respectively; and for cirrhosis, they were 91% and 81%, respectively.48 MRE has also been compared to TE, with one study showing that MRE produced similar results to ultrasound-based TE,49 while other studies found a higher technical success rate and improved diagnostic accuracy with MRE.30,50
As the prevalence of fatty liver continues to increase worldwide and locally, often linked to metabolic syndrome, it is important to assess the risk of progressive fibrosis leading to cirrhosis. All physicians who treat patients with fatty liver, particularly in primary care, internal medicine, and gastroenterology, should conduct risk classification studies.51,52 To this end, we suggest following the algorithm outlined in Figure 1.
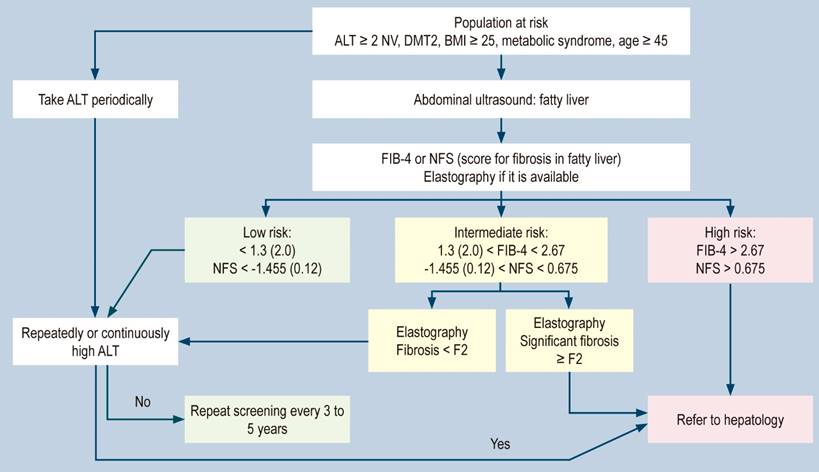
Figure 1 Liver risk algorithm in fatty liver.51 DMT2, Diabetes Mellitus Type 2; NV: normal value. Modified from: Dietrich CG et al. World J Gastroenterol. 2021;27(35):5803-5821
Treatment
Most current short-term clinical trials are designed to achieve histological resolution, improve nonalcoholic steatohepatitis (NASH) without fibrosis progression, improve fibrosis by at least one stage, or improve biochemical parameters.25,53,54 However, ideally, experts should focus on clinical outcomes such as a reduction in end-stage liver disease or liver transplantation due to fatty liver. Regarding type 2 diabetes, experts should also consider extrahepatic targets, such as cardiovascular or microvascular outcomes.54
Treatment for fatty liver disease is typically divided into four stages that progress in severity, as follows:
Weight loss through a combination of diet and exercise
Pharmacological measures
Endoscopic procedures
Surgical procedures
Weight Loss Following Diet and Exercise
Weight loss through diet and exercise is the fundamental and most critical treatment for all overweight (BMI > 25 kg/m2) or obese (BMI > 30 kg/m2) patients. For patients with simple steatosis, a weight loss of 5% to 7% of body weight is recommended, achieved at a rate of 0.5 to 1.0 kg per week. Patients with suspected or biopsy-proven NASH should aim for a 7% to 10% weight loss. If after reaching their weight loss target, the serum ALT level remains unnormalized (ALT < 20 for women and < 30 for men), patients should continue to lose weight until normalization is achieved.19,20,24,25,55,56
Diet
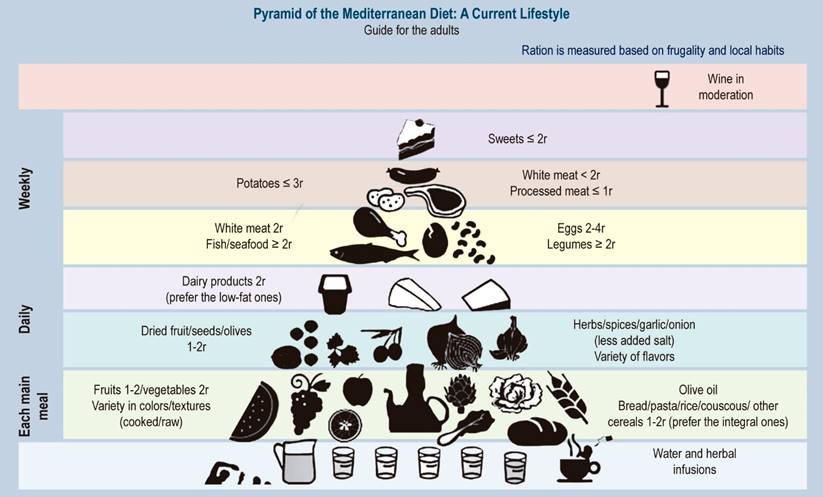
Based on current evidence, the best approach for weight loss is a combination of caloric restriction by at least 500-1000 kcal per day or a suitable diet low in saturated fat. However, adherence to these habits is crucial for successful weight loss.30,57) The most recommended diet for patients with fatty liver is the Mediterranean diet (MD), which is rich in fruits, fish, vegetables, nuts, and olive oil, among others.20,25,30,58,59 It has been shown to improve intrahepatic lipid content and insulin sensitivity.60 The constituent elements of the Mediterranean diet are shown in Figure 2.
Exercise
Experts recommend performing moderate-intensity aerobic physical activity for 150-200 minutes per week in three to five sessions,20,25,57 as it helps to maintain dietary weight loss and may have independent benefits on liver fat and histology.61 Studies suggest that exercise intensity and adherence to a training program are more important than the type of exercise performed, resulting in greater weight loss than education alone.61,62 Moreover, physical activity has been linked to survival benefits for patients with fatty liver, especially for aerobic exercise, with longer physical activity associated with lower mortality risks due to cardiovascular diseases.63,64
Pharmacological Measures
Pharmacological treatments are recommended by the American Association for the Study of Liver Diseases (AASLD) mainly to improve liver disease and should be limited to individuals with biopsy-proven NASH and fibrosis.19 The European Association for the Study of the Liver (EASL), the European Association for the Study of Diabetes (EASD), and the European Association for the Study of Obesity (EASO) guidelines recommend pharmacological treatment for patients with fatty liver with proven NASH and fibrosis ≥ F2, or patients at high risk of progression such as individuals with type 2 diabetes, metabolic syndrome, persistently high ALT, or high necroinflammation.20
NASH patients without diabetes
According to the AASLD, vitamin E is not recommended for treating fatty liver because the studies showing its usefulness did not include patients with diabetes mellitus or decompensated cirrhosis.19 Despite a meta-analysis that found no histological improvement with vitamin E,65 some studies suggest that doses of 800 IU/day may be beneficial. The largest randomized trial included in the meta-analysis (pioglitazone vs. vitamin E vs. placebo for the treatment of non-diabetic patients with NASH) found that patients who took vitamin E were more likely to improve their overall histological score (43% vs. 19%).66 Another report found that patients who received vitamin E had a more significant decrease in ALT values. (48% vs. 16%).67 The potential advantage of vitamin E may be related to its antioxidant properties. Therefore, vitamin E could be a reasonable treatment for patients with fatty liver and stage fibrosis ≥ 2 who do not have diabetes mellitus. However, it should be avoided in men with a high family history of prostate cancer. No dose ≥ 400 international units per day should be taken, as this has been inconsistently associated with increased all-cause mortality.
Patients with NASH and Diabetes
Metformin is considered the first-line drug. However, it does not improve steatosis or liver histology in patients with or without type 2 diabetes. Despite this, it has been found to promote moderate weight loss.(19,20,24,25,30,68-70
Thiazolidinediones, specifically pioglitazone, have been shown to improve liver biochemical and histological parameters in patients with nonalcoholic steatohepatitis (NASH).30 A meta-analysis demonstrated improvements in ballooning, lobular inflammation, and steatosis with thiazolidinediones, including fibrosis improvement with pioglitazone.71 Long-term treatment may be necessary to achieve clinical benefits since the discontinuation of pioglitazone can reverse improvements.72 In both diabetic and non-diabetic patients (type 2), pioglitazone has demonstrated histological reversal of NASH without worsening fibrosis.73 Pioglitazone acts on the peroxisome-proliferator-activated receptor gamma (PPARγ) in adipocytes, leading to adipose tissue remodeling and increased adiponectin secretion, resulting in reduced lipolysis, insulin resistance, and hepatic lipid storage.70,74 However, its use is limited in selected cases due to the potential side effects and risks, including weight gain, heart failure, and fractures.20
Glucagon-like peptide-1 receptor agonists. Food-induced secretion of intestinal hormones, glucagon-like peptide-1 (GLP-1), and gastric inhibitory peptide (GIP) are collectively referred to as incretins. These hormones can enhance insulin secretion in pancreatic β cells in response to glucose stimulation. GLP-1 has also been shown to suppress glucagon secretion, delay gastric emptying and intestinal glucose uptake, and is involved in the central regulation of food intake and satiety.75 For its part, GIP stimulates glucagon secretion. Glucagon-like peptide 1 receptor agonists (GLP-1 RA) are fundamental in treating type 2 diabetes and obesity because they induce weight loss, improve glycemic control, and produce beneficial changes in blood metabolism.30,70
Liraglutide effectively resolves NASH, reduces liver fat content, and decreases the likelihood of fibrosis progression.70,76,77 The approved dosage for antidiabetic use is up to 1.8 mg, while the recommended dose for weight loss is 3 mg.78
Semaglutide, at a dose of 0.4 mg once daily, has been shown to bring about histological resolution of NASH in patients with fatty liver disease and fibrosis.70,76,79,80 Compared to liraglutide, semaglutide is more effective in reducing body weight in individuals with type 2 diabetes and is approved for weight loss in patients without diabetes as well. At present, it is the most potent among the available drugs for weight loss, leading to the recent approval by the Food and Drug Administration (FDA) of a weekly subcutaneous dose of semaglutide at 2.4 mg for the management of chronic obesity in patients without diabetes.76,80
Sodium-glucose cotransporter-2 inhibitors (SGLT2 inhibitors) effectively reduce blood glucose levels and induce moderate weight loss by causing renal glucose loss and resulting in a caloric deficit.69,70,76,81 Recently, empagliflozin, an SGLT2 inhibitor, has been shown to significantly reduce liver fat content in patients with type 2 diabetes after 24 weeks of treatment.81 However, there are few studies on SGLT2 inhibitors that correlate with histological endpoints, and they have few patients and short duration.69,76 One study demonstrated improvement in the degree of steatosis, ballooning, and fibrosis with 25 mg per day of empagliflozin compared to placebo.82
Dipeptidyl peptidase-4 inhibitors prevent the endogenous degradation of incretin, thereby prolonging the endogenous action of GLP-1. However, in clinical trials, dipeptidyl peptidase-4 inhibitors (DPP4i) have shown negative results for treating NAFLD,69,70,76 except for vildagliptin, which has been shown to reduce liver fat.
Combination therapy involving different classes of medication has shown promise in the treatment of fatty liver. GLP-1 RA and SGLT2i have proven effective in reducing cardiovascular risk and are recommended as first-line therapies for patients with type 2 diabetes and established or high-risk cardiovascular disease (CVD). Possible combinations include semaglutide/SGLT2i or low-dose pioglitazone combined with GLP-1 RA or SGLT2i.69,70,76
Other Treatments in General
The primary benefit of statins is the reduction of cardiovascular risk. However, pilot studies have also suggested that atorvastatin may have a beneficial effect on aminotransferase levels in patients with NAFLD,30,69,76 without any associated hepatotoxicity.83
Omega-3 fatty acids have been shown to improve hepatic steatosis and AST levels in a meta-analysis on fatty liver.84 However, when the analysis was limited to data from randomized trials, only improvement in hepatic steatosis was observed with the use of omega-3 fatty acids.
Table 1 provides a summary of the fundamental aspects of treatments for diabetes.
Table 1 Diabetes treatment70
| Medication | Hepatic fat | NASH/NAS Activity | Changes in weight | Cardiovascular effects | Side effects |
| Metformin | No changes | No changes | No changes | Potential benefit in ACD | Common gastrointestinal effects (diarrhea, nausea) Lactic acidosis Vitamin B12 deficiency |
| Pioglitazone | Decrease | Improvement | Increase | Potential benefit in ACD Increased risk of HF | Weight gain Fluid retention Increases the risk of fractures Increases bladder cancer |
| SGLT2i Empagliflozin Canagliflozin Dapagliflozin | Decrease | Unknown | Decrease | ACD benefit of empagliflozin and canagliflozin HF benefit of empagliflozin, canagliflozin, and dapagliflozin | Risk of DKA from surgery Risk of bone fractures with canagliflozin Genitourinary infections Volume depletion Increases LDL |
| GLP-1 RA Lixisenatide Liraglutide Semaglutide Dulaglutide Albiglutide Exenatide | Decrease | Improvement | Decrease | ACD benefit of liraglutide, semaglutide, and dulaglutide | FDA indicates a risk of thyroid tumors in rodents Common gastrointestinal effects (diarrhea, nausea, vomiting) Pancreatitis |
| DPP4i Saxagliptin Alogliptin Sitagliptin Vildagliptin Linagliptin | No changes | Unknown | No changes | Potential HF risk of saxagliptin | Pancreatitis Joint pain |
GLP-1 RA: glucagon-like peptide-1 receptor agonists; DKA: diabetic ketoacidosis; ACD: atherosclerotic cardiovascular disease; HF: heart failure; DPP4i: dipeptidyl peptidase-4 inhibitors; SGLT2i: sodium-glucose cotransporter-2 inhibitors; LDL: low-density lipoprotein; NAS: NAFLD activity score; NASH: nonalcoholic steatohepatitis. Modified from: American Diabetes Association. Diabetes Care. 2021;44(Suppl 1):S111-S124.
Future of Treatment for Patients with Fatty Liver and Type 2 Diabetes
Currently, double and triple agonists of GLP-1, GIP, and glucagon receptor in combination are being tested in phase 2 and 3 clinical trials for treating obesity and type 2 diabetes.75 Tirzepatide, a dual GLP-1/GIP receptor agonist, demonstrated an average reduction in body weight of 9.5 kg (11.0%) with a weekly dose of 15 mg.85,86 Another drug, Thesamorelin, a growth hormone-releasing hormone analog indicated for treating lipodystrophy in HIV, showed selective reductions in visceral and hepatic fat and weight loss, which has led to an ongoing study of fatty liver.69,86 Resmetirom, a selective thyroid hormone receptor β (THR-β) agonist, was designed to improve NASH by increasing liver fat metabolism and reducing lipotoxicity.69,86 In addition, Lanifibranor, a pan-PPAR agonist, achieved an improved combined resolution of NASH and fibrosis in a dose-dependent manner in adults with type 2 diabetes and NASH.69,86
Endoscopic Procedures
Over the past ten years, the US FDA has approved six types of endoscopic bariatric and metabolic therapies (EBMT) as an alternative to bariatric surgery. Some of these therapies are reversible and have a lower cost and risk of complications than surgery.87,88 Essentially, EBMT involves placing intragastric balloons or endoscopic intragastric sutures. The best-known options are the Orbera intragastric balloon system, the Obalon balloon system, and the OverStitch endoscopic suturing system (Apollo Endosurgery) for endoscopic sleeve gastroplasty (ESG).87 These procedures are reserved for patients who do not achieve weight loss through diet, exercise, and medications and are at high risk of fibrosis progression. Endoscopic bariatric procedures have been shown to result in higher and sustained weight loss percentages, with regression of hepatic steatosis, steatohepatitis, and fibrosis occurring in 30% of patients.89,90
Surgical Procedures
Bariatric surgery is recommended for patients with NASH or advanced fibrosis without decompensated cirrhosis who have not achieved their weight loss goals after appropriate follow-up.91 A systematic review reported improvement in steatosis in eighteen studies, decreased inflammation in eleven studies, and an improved fibrosis score in six studies.92 However, four studies showed a deterioration in fibrosis, emphasizing the need for proper postoperative follow-up for all patients.
Bariatric surgery offers a viable option for achieving sustained weight loss and improving the histological components of NAFLD, as well as improving type 2 diabetes.93) In addition, it has been shown to improve cardiovascular outcomes in both diabetic and non-diabetic obese patients.94 However, bariatric surgery can also result in peri- or postoperative complications that should be taken into account.92,95
Finally, the current pillars of treatment are summarized in Figure 3.
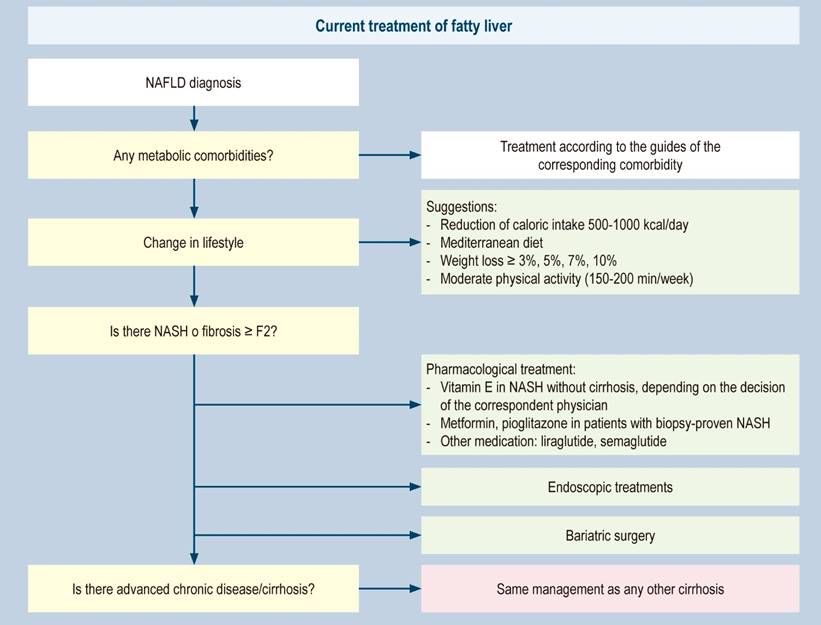
Figure 3 Treatment of fatty liver57. Modified from: Paternostro R et al. J Intern Med. 2022;292(2):190-204.











 texto en
texto en