Introduction
Interleukin 17 (IL-17) is a proinflammatory cytokine that has been linked to the pathogenesis of inflammatory bowel disease (IBD).1 Selective inhibitors of IL-17, such as secukinumab (a monoclonal antibody approved for treating psoriasis, psoriatic arthropathy, and ankylosing spondylitis [AS]), have been paradoxically associated with exacerbations or the development of IBD. This seems to be due to the protective effect of IL-17 against inflammation by inhibiting the Th1 response and maintaining the epithelial barrier of enterocytes, thus preserving intestinal homeostasis.2
Presentation of the case
A 43-year-old female patient with a medical history of ankylosing spondylitis (AS) since the age of 20, fibromyalgia, arterial hypertension, hypothyroidism, and latent tuberculosis treated in 2019 presented to the hospital with symptoms of diffuse abdominal pain and multiple bloody diarrheal stools (more than fifteen times a day) for a week. The patient had been treated with etanercept, adalimumab, abatacept, and since 2017, with secukinumab. On admission, the patient was tachycardic, dehydrated, afebrile, and had lower abdominal pain without signs of peritoneal irritation. Laboratory tests showed no alterations in blood count, and an ultrasound and tomographic study revealed wall thickening of the right colon with mucous enhancement, multiple mesenteric lymphadenopathies, and some free fluid at the bottom of the sac (Figure 1). Colonoscopy showed edema, erythema, mucosal friability, loss of vascular pattern, and ulcerations covered by fibrin from the rectum to the cecum, which are consistent with extensive ulcerative colitis (Figure 2 ).
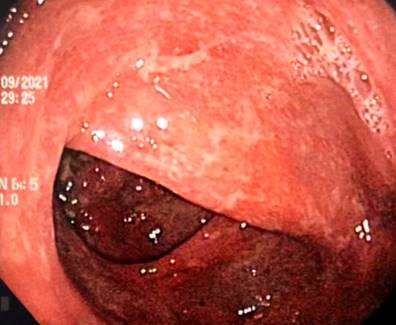
Figure 1 Tomographic findings. Thickening of the walls of the ascending colon with mucous enhancement (arrow). Source Authors’ archive.
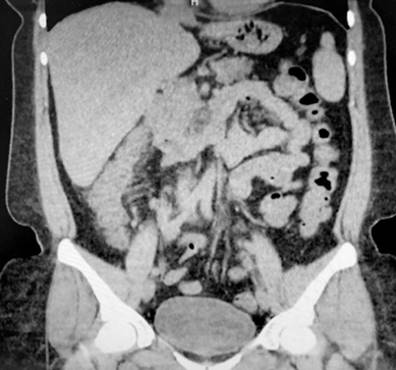
Figure 2 Endoscopic findings. Edema, erythema, loss of vascular pattern, and ulcerations covered by fibrin. Source: Authors’ archive.
Based on the patient’s symptoms and endoscopic findings, a diagnosis of severe ulcerative colitis was made, and treatment with intravenous corticosteroids, oral 5-aminosalicylic acid (5-ASA in granules), and enemas was initiated. The pathology report indicated that the patient had inflammatory bowel disease suggestive of severe ulcerative colitis without dysplasia or metaplasia. The patient’s infectious profile showed only positive results for CMV IgG, normal liver function, and a fecal calprotectin level above 1000 ng/mL. Fortunately, the patient responded well to treatment and experienced a resolution of her symptoms. As a result, the corticosteroid treatment was gradually discontinued, and the patient was switched to tofacitinib in consultation with the rheumatology team.
Discussion
Secukinumab is a human monoclonal antibody that specifically blocks interleukin 17A (IL-17A),3 an inflammatory regulation molecule linked to the development of autoimmune diseases such as ankylosing spondylitis (AS), psoriatic arthritis (PA), and psoriasis. Additionally, murine studies suggest a role for IL-17A in maintaining gastrointestinal homeostasis and tissue repair by stimulating mucin production, which strengthens the bond between claudin and occluding proteins5 and maintains intestinal barrier integrity.4 Although spondyloarthropathies such as AS and PA (treated with secukinumab) share overlapping characteristics with inflammatory bowel disease (IBD), their immune mechanism is not well understood. It is noteworthy that at least 50% of patients with AS or PA exhibit histological changes of inflammation in colon samples, and approximately 7% may develop Crohn’s disease or ulcerative colitis,6 which suggests that they have a threefold higher risk of developing IBD compared to the general population.7,8
Blocking IL-17A, which is used as a treatment for AS, may lead to a possible deterioration of the intestinal epithelial barrier, which could initiate or worsen the phenotypes of IBD, such as ulcerative colitis or Crohn’s disease.3
It has been reported that up to 7.8% of patients receiving secukinumab may present gastrointestinal symptoms associated with its administration.9 However, most of them do not develop objective evidence of IBD or require treatment discontinuation. The incidence of new cases of IBD following secukinumab administration has been reported between 0.2% and 0.7%.7,10,11
Studies have reported that up to 7.8% of patients treated with secukinumab may experience gastrointestinal symptoms related to its use.9 However, the majority of these cases do not result in the development of clear evidence of IBD or require treatment discontinuation. The incidence of new-onset IBD following secukinumab treatment has been estimated to range from 0.2% to 0.7%.7,10,11
While the incidence of IBD in patients treated with secukinumab is low, it is still recommended to carefully investigate the patient’s family history of IBD or gastrointestinal symptoms before starting treatment.7 Patients should be informed of the possibility of developing gastrointestinal adverse events. As some may present subclinical IBD, fecal calprotectin is suggested, and patients with normal values may be considered for treatment, although close monitoring for the appearance of gastrointestinal symptoms is essential. Those with elevated values should be evaluated to rule out an IBD diagnosis. For patients with active IBD, treatment initiation is contraindicated. For patients with a known diagnosis of IBD in the quiescent phase, other therapeutic options should be considered.12 Currently, there are no known reported cases of secukinumab-induced or exacerbated IBD in Colombia.
Conclusions
Although the incidence of IBD in patients treated with IL-17 inhibitors is low, it is important to note that a significant percentage of patients receiving this group of medications may develop gastrointestinal adverse events that require monitoring and follow-up.
Performing a thorough evaluation of family history, previous symptoms, and fecal calprotectin levels before starting treatment is a proposed strategy to reduce the risk of developing IBD or worsening existing symptoms.











 text in
text in 



