Introduction
Cystic intestinal pneumatosis (CIP) or pneumatosis cystoides intestinalis (PCI) was described by Du Vernoy in 1730.1 It is a rare but well-identified clinicopathological condition characterized by the presence of bullae or aerial cysts in the subserosa of the small intestine and the submucosa of the colon,2 especially transverse, descending, and sigmoid, with a higher proportion in men (2.4:1).3 It is usually asymptomatic or with nonspecific symptoms such as abdominal pain, diarrhea, rectorrhagia, obstruction, flatulence, and rectal tenesmus,4 and may be an incidental finding in colonoscopies and radiological studies. Histopathologically, it is characterized by cystic structures lined with histiocytes, giant cells, and granulomatous inflammatory reaction with eosinophils and elastosis.5
Etiologically, it can be primary or idiopathic (15%) and secondary, associated with other diseases (85%).3,6 According to its clinical severity, it is classified as potentially lethal intestinal pneumatosis and benign intestinal pneumatosis (BPI).4 The rupture of cysts can cause pneumoperitoneum, air in the omentum, or pneumoretroperitoneum in less than 3% of cases,7 conditions that require either conservative or surgical management.
We present the case of a 23-year-old patient with four months of diarrhea and intermittent rectorrhagia. On colonoscopy, he was found to have cystic pneumatosis involving the sigmoid and descending, associated with acute infectious colitis. Computed tomography (CT) of the abdomen confirmed CIP and encapsulated pneumoperitoneum. After eight months of follow-up, he underwent conservative treatment with antibiotics and improved without surgery.
Clinical case
This is a 23-year-old male patient of urban origin, with four months of episodes lasting three days consisting of an increase in the frequency of bowel movements to three a day and one at night, of a liquid consistency, with mucus and blood, associated with abdominal distension, flatulence, pushing, and rectal tenesmus, in addition to the loss of 7 kg of weight. He had a history of isotretinoin treatment for acne for four years and a vaccine for 2019 coronavirus disease (COVID-19) with the first dose of the Pfizer vaccine. The stool test reported blastocystis hominis, Sudan III, in negative fecal matter and positive occult blood. It was treated as amoebic colitis with metronidazole and probiotic, rapidly improving diarrhea, but the patient had a symptomatic relapse eight days later.
On physical examination, vital signs were normal. He weighed 67 kg, was 1.78 m tall, and had a body mass index (BMI) of 21.15 kg/m2, with no relevant findings. Laboratory tests reported C-reactive protein (CRP < 6.0), non-reactive human immunodeficiency virus (HIV), fecal calprotectin < 15, and carcinoembryonic antigen < 0.5, with normal liver and kidney profile. With a diagnosis of prolonged diarrhea and rectorrhagia under study, the patient underwent an upper endoscopy that led to the diagnosis of acute erosive gastritis and chronic diffuse corporoantral gastritis, Helicobacter pylori (-), and duodenal biopsies that ruled out celiac disease. In a colonoscopy, the rectal and colon mucosa between the splenic angle and the terminal ileum were normal, but there were multiple cystic, rounded, dark reddish confluent lesions up to 1.5 cm in the sigmoid and descending, with eroded overlying mucosa and soft on palpation with clamp (Figures 1A and B). When in doubt about the liquid or air content of the cysts, a fine needle puncture was performed in one of the largest cysts, with no fluid outlet and complete collapse of the cyst (Figures 1C and D). The same situation occurred when taking a biopsy of the wall of another of the cysts, which also had collapsed (Figure 1E and F). These findings are compatible with colonic cystic pneumatosis associated with acute erosive colitis of probable infectious or parasitic etiology. There was no abdominal pain, bloating, or other symptoms at the end of the colonoscopy.
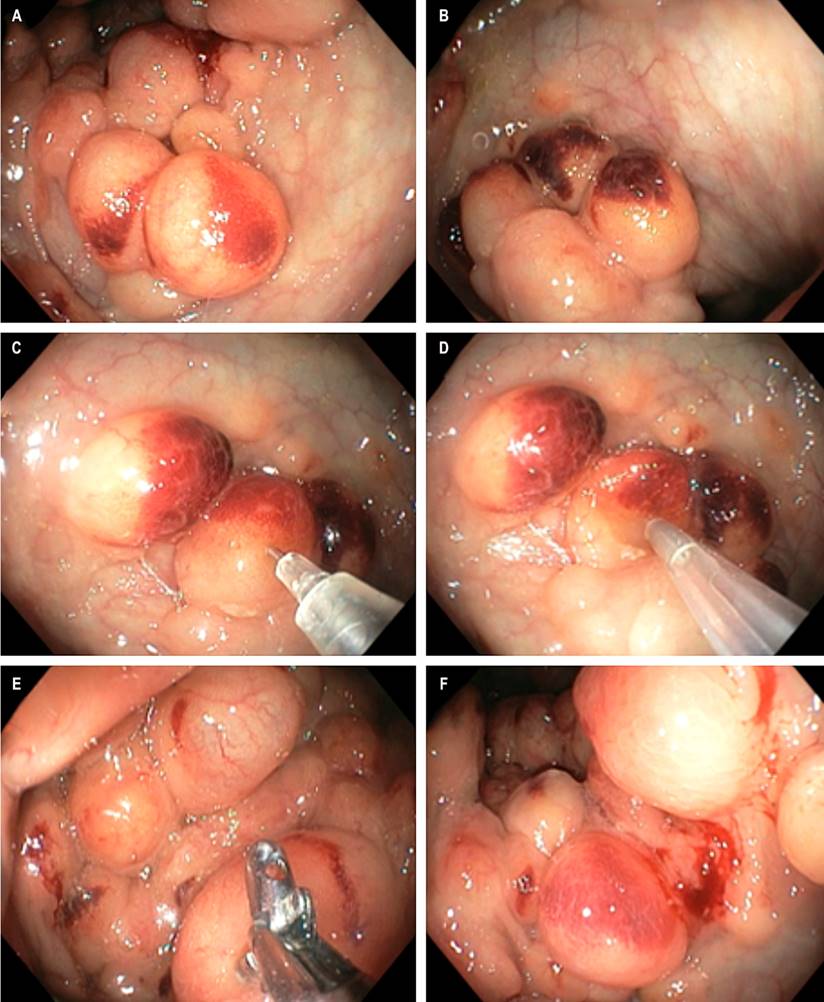
Figure 1 Colonoscopy. A and B. Multiple cluster cystic images in the sigmoid and descending, with eroded mucosa overlying by acute colitis. C and D. Fine needle puncture and air suction, no liquid outlet, with complete bubble collapse. E and F. Biopsy of the cyst wall with bubble collapse. Source: Authors’ archive.
CT of the abdomen with contrast showed multiple thin-walled air bubbles, without extravasation of contrast medium, predominantly of perihepatic distribution and, to a lesser degree, left subphrenic (Figures 2A and B), with multiple extraluminal air bubbles, located primarily in the descending and sigmoid colon walls (Figures 2C and D). Some of them were in clusters, adhered and adjacent to an “encapsulated pneumoperitoneum chamber” of approximately 160 mm larger diameter next to the splenic angle (Figures 2E and F), compatible with the endoscopic findings of PCI.
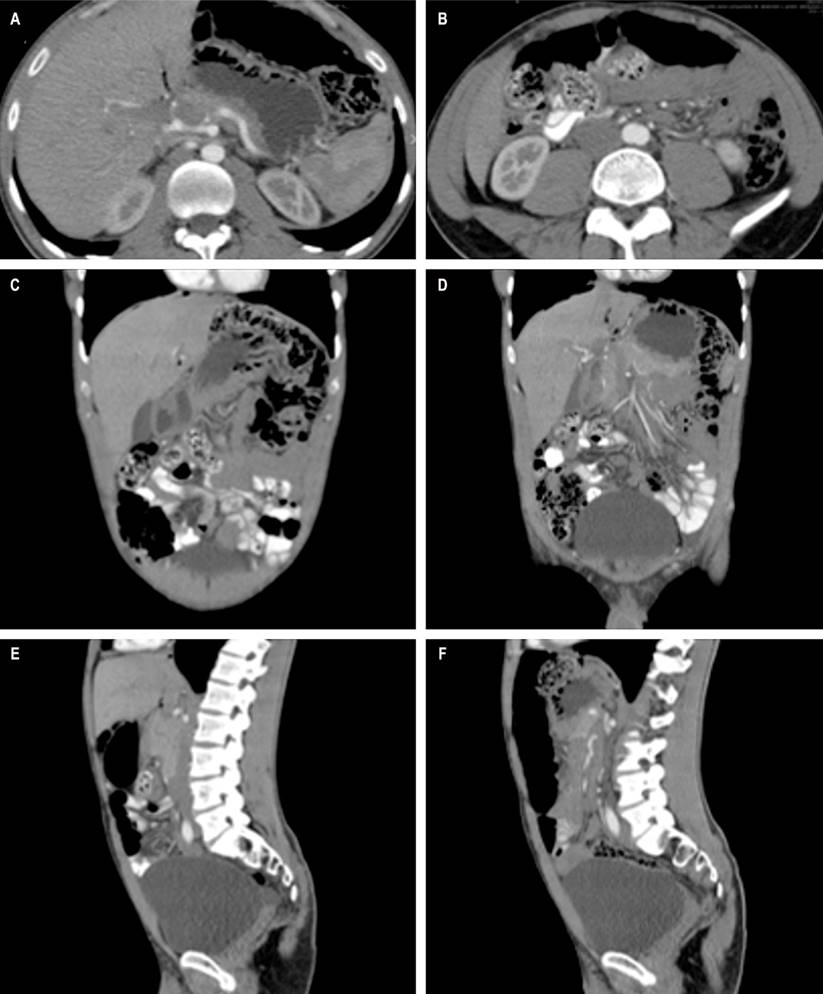
Figure 2 CT scan of the abdomen. A and B. Sagittal section. Cluster colonic cystic pneumatosis, with multiple thin-walled air bubbles, without extravasation of the contrast medium. C and D. Coronal section. Multiple extraluminal air bubbles located in the walls of the descending and sigmoid colon. E and F. Axial cut. Pneumoperitoneum in the anterior abdomen, 16 cm in diameter next to the liver and in continuity with the bubbles of the splenic angle of the colon. Source: Authors’ archive.
Cystic wall histopathology reported a chronic hemorrhagic inflammatory process with focal erosion, fibroblast proliferation with angiectasias, and multifocal reactive neovascularization. Comprehensive multidisciplinary analysis of PCI with asymptomatic pneumoperitoneum led to conservative treatment with tinidazole 500 mg twice a day for fifteen days, rifaximin 550 mg twice a day for fifteen days, pinaverio/dimethicone 100/200 mg twice a day for 30 days, esomeprazole 40 mg/day for 30 days, and insoluble fiber 20 mg/day for 30 days, with a relevant symptomatic improvement and disappearance of diarrhea and rectorrhagia, which was maintained until the 8-month follow-up.
Discussion
Pneumatosis cystioides intestinalis is characterized by the presence of cysts with gas in the intestinal submucosa or subserosa and occurs in the large intestine in 46% of cases (especially in the sigmoid), the small intestine (27%), the stomach (5%), the small intestine and colon (7%), and very occasionally in the duodenum and rectum.3,8
Among the attributed pathophysiological mechanisms, there is inflammation, physical damage of the intestinal mucosa, nutritional imbalance, dysbiosis, gastrointestinal dysmotility, and immune dysfunction associated with predisposing factors such as surgeries with intestinal anastomosis, chemotherapy (rituximab, cetuximab), scleroderma, connective tissue diseases, lung disease, use of medications for diabetes (voglibose, acarbose), or use of trichloroethylene and inhibitors tyrosine kinase (sunitinib).8
Its etiology is imprecise and includes theories such as the “mechanical” one because intestinal obstruction increases intraluminal pressure and promotes cyst formation; “pulmonary” for chronic obstructive pulmonary diseases (COPD, asthma), where rupture of alveoli and subsequent pneumomediastinum cause air dissection along the aorta and mesenteric vessels, terminating air in the intestinal wall; “bacterial”, because of the gas produced by intestinal bacteria that cross the mucosa and reach the submucosa; “chemical and nutritional deficiency”, due to bacterial fermentation of carbohydrates with increased production of gas that penetrates the intestinal wall; “immunological” or intestinal toxicity (use of chemotherapy and hormonal therapy) that generates air passage due to functional and organic dysfunction of the intestinal wall.9
The diagnosis can be suspected upon the finding by optical colonoscopy (OC) of intramural cysts in violet clusters, which simulate a liquid content due to the inflammation of the overlying mucosa, as well as by fine needle puncture, in which the collapse of the cyst is observed without a fluid outlet, or by appreciating the exit of bubbles from the cyst when taking biopsies underwater.6
Virtual colonoscopy and CT of the abdomen confirm the diagnosis by finding cysts that look like bunches of grapes or honeycombs in the intestinal wall, which show gas content in reconstructions with lung window. In cases of severe forms of PCI, intestinal dilation, arterial or venous occlusion, ascites, and porohepatic or portomesenteric venous gas can be observed.4,9
PCI is usually asymptomatic (even with pneumoperitoneum) or may have nonspecific symptoms. In these cases, treatment may be conservative through observation and follow-up,4,6,8 hyperbaric oxygen therapy,10 antibiotics (metronidazole, tinidazole, rifaximin, quinolones), Bifidobacterium probiotics, endoscopic therapy such as fine needle aspiration6,11 or high-frequency electrosurgical resection of cystic walls or sclerotherapy. The use of plasma argon is not recommended due to possible explosion by methane,8 and neither change of chemotherapeutic (cytostatic type or targeted molecular therapies and tyrosine kinase inhibitors for metastatic esophageal, pulmonary, renal, or other tumor pathology).12
In these patients, pneumoperitoneum should be considered an incidental sign. Other etiologies of non-surgical pneumoperitoneum should be ruled out, such as retained postoperative air or thoracic, gynecological, or abdominal causes, which in asymptomatic patients can resolve spontaneously so that unnecessary surgeries are avoided.13
Surgical intervention should be considered in symptomatic patients with abdominal pain, intestinal obstruction, bleeding, peritoneal irritation, acidosis, portal gas, and pneumoperitoneum, given the potential for ongoing ischemic colitis, necrosis, or intestinal obstruction.2,14 The decision to undergo surgery should be based on the presence of associated risk conditions, a physical examination suggesting peritonitis, analytical predictors of poor prognosis (pH < 7.3, bicarbonate < 20 mL/L, lactate > 2 mmol/L, serum amylase > 200 U/L) or the presence of portal venous gas.15 Pneumoperitoneum alone is neither a predictor of severity nor an indicator of emergency surgery.13
Surgically, PCI can be found in the sigmoid with chronic volvulus causing intermittent partial obstruction or perforation with peritonitis, which requires sigmoidectomy.14,16 If no visceral perforation, peritonitis, or indication of bowel resection is found, surgery can be terminated, and conservative management can continue according to the etiological cause.13
There are no management guidelines for PCI; in most cases, it is done conservatively. In this patient, the incidental finding of pneumoperitoneum with no symptoms and a normal physical and paraclinical examination, without suspicion of peritonitis, allowed a non-surgical treatment based on antibiotics for the associated acute infectious colitis, and a symptomatic remission was obtained until the eighth-month follow-up. Colonoscopic, histopathological, and tomographic follow-ups will be carried out one year after diagnosis.
Conclusions
In patients with an incidental finding of PCI, diagnostic and therapeutic decisions should be rapid, based on multidisciplinary history analysis, physical examination, and biochemical, endoscopic, histopathological, and imaging analysis.
The finding of pneumoperitoneum in these patients should be an alarm sign but not an absolute indicator of surgical intervention.











 text in
text in 



