Introduction
Obesity is a public health problem with high morbidity and mortality. It has been shown that a 5 kg/m2 increase in body mass index (BMI) above 25 kg/m2 is associated with an increased risk of all-cause mortality1. It is deemed a chronic and difficult-to-manage disease, which results in multiple metabolic, cardiovascular, joint, and psychosocial complications. Furthermore, it has been described that achieving a sustained weight loss of 5% to 10% can prevent and reduce cardiovascular risk and other complications in people with obesity2.
Initial management includes conservative measures such as caloric restriction, exercise, and behavioral changes. Pharmacotherapy is recommended in patients who fail to lose weight, and the surgical approach is reserved for highly obese people3. The intragastric balloon (IGB) is an intermediate step between medical and surgical management4,5 and an attractive choice because it is considered a temporary (less than six months), reversible, minimally invasive, safe, and effective weight loss method6-8. A mean total body weight loss of 15.5% has been proven in overweight or obese adults one year after removal7.
Currently, IGB models include those with fluid or air content; the most widely used are fluid-filled due to their lower rate of complications9. When placed, the IGB floats freely in the stomach, with a multifactorial mechanism of action and physiological and neurohormonal changes, leading to increased satiety and decreased gastric reservoir capacity and food intake4. It can be kept in the stomach for six months, and a new generation of IGB allows up to 12 months10. Today’s most used IGBs include Orbera, Spatz 3, Reshape Duo, Bariatrix, Elipse, and Heliosphere. There is no precise indication in the literature regarding which to employ, so the best option is chosen according to the physician’s criteria and experience in each technique11. In Colombia, there is little information about the results of implementing fluid-filled IGB in low- and moderate-risk obesity (BMI of 30-40 kg/m2).
This study aims to evaluate the implementation of fluid-filled IGB in individuals with low- and moderate-risk obesity (BMI of 30-40 kg/m2) regarding weight loss, safety, and tolerance at 4, 6, and 12 months of treatment.
Materials and methods
Study design and data extraction
A prospective descriptive observational study was conducted using convenience sampling that took as the source population patients with low- and moderate-risk obesity (BMI of 30-40 kg/m2) at Clínica Palermo from January 1, 2019, to December 31, 2020. Clínica Palermo is a tertiary referral hospital and a national benchmark in gastroenterology. The study population consisted of patients ≥18 years of age, refractory to conservative treatment, and participants in a weight loss program. Individuals with a BMI > 40 kg/m2 or with contraindications for IGB were excluded. All patients were informed and signed the informed consent.
Data collection
The medical records and the official report of the procedure performed were used as the primary source of information, collecting sociodemographic and clinical variables on admission. The variables of sex, age, initial and final weight, weight loss percentage, and side effects were analyzed. Complications were considered adverse effects attributable to IGB after two weeks of insertion, found during outpatient follow-up. Standard methods for quantifying weight loss, such as BMI and weight loss percentage, were used.
Procedure
The preprocedural weight loss protocol consisted of multidisciplinary outpatient follow-up (with a gastroenterologist and nutritionists). The IGB implantation was initially managed with a hypocaloric diet (1,000 cal/day) and physical activity.
One hundred nine patients were included and underwent endoscopic implantation of Apollo Endosurgery’s Orbera, Allurion’s Elipse, and Spatz 3 IGBs. Each patient needed a single IGB with a filling capacity of 500-700 mL (Figure 1). These procedures were performed under sedation by anesthesiology without endotracheal intubation.
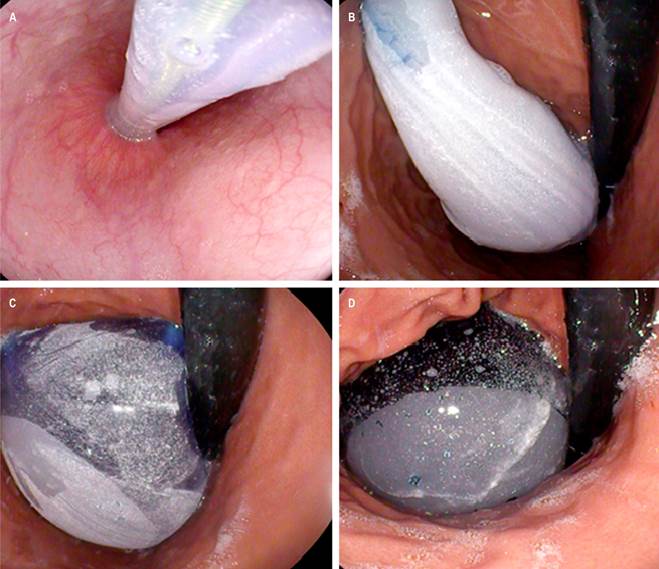
Figure 1 Fluid-filled IGB implant. A. Endoscopic view of the positioning of the IGB during implantation. B. Endoscopic view of the IGB filled with fluid. C. Endoscopic view of the IGB in an adequate position, completing the fluid filling. D. Endoscopic view of the IGB after filling is complete, with no fluid leak. Authors’ archives.
Extraction was also performed under sedation in 106 patients without endotracheal intubation. Gastroscopes with a 2.8 mm working channel and standard accessories (needle catheter, foreign body forceps, and polypectomy loops) were used (Figure 2). Three patients presented with spontaneous expulsion (Allurion’s Elipse).
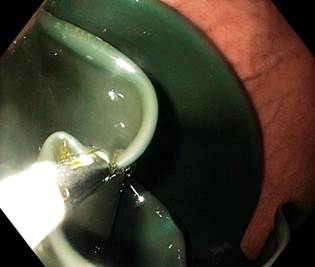
Figure 2 Endoscopic removal of the IGB. Endoscopic view of the empty balloon after suctioning the fluid. Authors’ archives.
Periodic follow-ups with gastroenterologists were conducted to assess efficacy and tolerance. Proton pump inhibitors (PPIs) were prescribed during the IGB stay, along with antiemetics and analgesics, for the first two weeks. Weight monitoring was performed in all patients before IGB implantation, at each follow-up control, and upon extraction.
Definitions
The ideal weight was considered 18.7-24.9 kg/m2 for all adults, regardless of age (12), and an effective weight loss percentage of at least 10% of excess weight (13).
Statistical analysis
The database was prepared in Excel v. 2019. We completed the missing data with additional reviews of the information sources, and in the end, only complete data were analyzed. Data processing was conducted in the social sciences program SPSS v. 25.0. The arithmetic mean was used for the descriptive analysis of quantitative variables. At the same time, absolute and relative frequencies were employed for qualitative variables.
Ethical considerations
This study was approved by the ethics and research committee of Clínica Palermo, Bogotá, Colombia. The primary sources of information included clinical records. Its design met the requirements in Resolution 8430/1993 issued by the Colombian Ministry of Health, so it was regarded as a low-risk study. Confidentiality of the information collected was guaranteed. All patients were informed and signed the informed consent. None of the records had sensitive information about the identity of patients.
Results
Of the 109 operated patients, eighty-two were women (75.22%). The average weight of the patients was 87.22 kg, with an average BMI of 31.59 kg/m2. The main comorbidities at the beginning of treatment were high blood pressure (HBP; n: 23, 21.1%), type 2 diabetes (n: 18, 16.51%), mechanical joint pain in the lower limbs (n: 15, 13.76%), and obstructive sleep apnea-hypopnea syndrome (OSAHS; n: 9, 8.25%) (Table 1). Three brands of fluid-filled balloons were used (Orbera, n: 103; Spatz 3, n: 3; and Elipse, n: 3).
Table 1 Characteristics of patients treated with IGB at the beginning
| Parameter | Statistics |
|---|---|
| Female sex (n: 82) | 75.22% |
| Average age | 33.44 years |
| Comorbidities | |
| HBP (n: 23) | 21.10% |
| Type 2 diabetes (n: 18) | 16.51% |
| Joint pain (lower limbs) (n: 15) | 13.76% |
| OSAHS (n: 9) | 8.25% |
Table prepared by the authors.
The IGB was implanted for an average of 8.2 months, using fluid-filled devices in all cases. The average weight loss varied significantly between Elipse and Orbera, although there were very few cases with this first brand. Globally, a reduction of the average BMI to 27.71 kg/m2 was achieved. The average weight loss had significant differences in the analysis by month and brand, respectively: Elipse: four months (-4.6 kg), Spatz 3: three months (-7 kg), Orbera: six months (15.2 kg), Orbera: 12 months (19.7 kg). Table 2 shows the baseline anthropometric variables of the included patients and Table 3 after IGB implantation.
Table 2 Baseline demographic and anthropometric characteristics of the patients on the day of IGB insertion
| Parameter | Orbera (n = 103) | Spatz 3 (n = 3) | Elipse (n = 3) |
|---|---|---|---|
| Female sex, n (%) | 80 (77.66) | 2 (66.66) | 2 (66.66) |
| Age, mean (SD) | 33.2 (5.2) | 36.4 (3.32) | 38.5 (4.6) |
| Starting weight, mean (SD) | 87.36 (8.31) | 85.38 (7.17) | 84.25 (5.92) |
| Excessive weight, mean (SD) | 18.27 (3.46) | 19.62 (4.62) | 19.1 (4.03) |
| BMI, mean (SD) | 31.34 (3.56) | 36.78 (7.23) | 34.98 (5.92) |
IGB: intragastric balloon; SD: standard deviation; BMI: body mass index. Table prepared by the authors.
Given that the population sample was small and that this is a descriptive study, we could not evaluate statistically significant differences between assorted brands of IGB. However, a trend toward more significant weight loss was seen in periods of IGB implantation greater than six months (Table 3).
Table 3 Anthropometric characteristics of the patients on the day of IGB removal
| Parameter | Orbera, 12 months (n = 58) | Orbera, 6 months (n = 45) | Spatz 3 (n = 3) | Ellipse (n = 3) |
|---|---|---|---|---|
| IGB implantation time, months, mean (SD) | 12 (0.8) | 6 (1.1) | 3 (0.2) | 4 (0.5) |
| BMI reduction (kg/m2) (mean ± SD) | 8.12 ± 3.76 | 7.42 ± 3.34 | 3.3 ± 0.62 | 2.1 ± 0.3 |
| Weight loss (kg), mean, (SD) | 19.7 (6.3) | 15.2 (4.32) | 7 (1.83) | 4.6 (0.23) |
| Excessive weight loss percentage (mean ± SD) | 26.2 ± 2.3 | 22.4 ± 1.84 | 10.56 ± 0.92 | 8.5 ± 1.86 |
IGB: intragastric balloon; SD: standard deviation; BMI: body mass index. Table prepared by the authors.
The balloon was removed after two months in three patients (2.75%), in two (1.83%) due to intolerance (abdominal pain), and one due to acute appendicitis (0.91%). No cases of acute pancreatitis or digestive bleeding were documented. There were no complications at the time of removal, neither with the procedure nor the sedation.
Discussion
The fluid-filled IGB is a less invasive option for managing low- and moderate-risk obesity, particularly in cases of BMI greater than 25 kg/m2, without optimal results after initial medical management14,15. Beyond this, it does not permanently interfere with the gastric anatomy or the size of the gastric volume due to interventions such as sutures, stomas, and thermal destruction of the mucosa, among others, used in other methods5,11,16. The main complications described in the literature include deflation or migration (up to 28.9% of cases), followed by minor side effects (0.2%-27%), nausea, and vomiting (18%)(16, 17). The present study found an overall frequency of complications of less than 3% in two patients (1.83%) due to intolerance (abdominal pain), approximating that described in the study by Sander et al.18, in which there was an early removal of the IGB in 3% of the cases. Intolerance is characterized by persistent emesis for extended periods associated with abdominal distension, which can lead to the patient’s dissatisfaction or lack of motivation19. This symptomatology is more attributable to all gas-filled IGB and less frequent in IGB with fluid content11. In our study, early removal of the IGB was required due to the risk of electrolyte imbalance, dehydration, and kidney failure in these patients. The percentage of intolerance found is as reported in the literature without finding any fundamental predisposing characteristic.
Other less frequent complications include gastric perforation, overfilling, intestinal obstruction, gastric dilatation and impaction20. None of these complications was reported in the population of our study, so their overall incidence is considered acceptable. One patient (0.91%) in the study had acute appendicitis. The manifestation of appendicitis is rare, as described in the literature5, and may be a coincidence rather than a direct cause related to the IGB or the procedure. This study corroborates that the manifestation of appendicitis and the complications associated with the procedure requires the removal of the IGB.
Weight loss is the primary outcome of interest beyond comparison when evaluating the IGBs. The results in weight loss are heterogeneous in the different studies, with variable results concerning multiple factors11. Most studies establish as selection criteria patients with a BMI greater than or equal to 40 kg/m2, with variable weight loss at six months of 17-21 kg21,22; however, few studies specifically assess the efficacy of fluid-content IGB in patients with low- and moderate-risk obesity in periods ranging from 6 to 12 months. In a retrospective study by Fittipaldi-Fernández et al.23, the implementation of the IGB was evaluated in 5,874 subjects with overweight and any degree of obesity, with a predominant population of women (n = 4,081; 74.96%). According to the subgroups, 371 (6.81%) were overweight (BMI: 25-29.99 kg/m2), and 1,848 (33.94%) were grade I obese (BMI: 30-34.99 kg/m2), together totaling 37.7% of the total sample. Overall, there was a weight loss of 19.13 ± 8.86 kg; according to the obesity groups, there was a weight loss of 12.83 ± 4.51 kg and 16.2 ± 6.42 kg in the overweight and grade I obesity groups, respectively. In our cohort, we found a more significant weight loss at 12 months (19.7 kg) and a mean decrease in BMI of 3.88 kg/m2. This figure is close to that described by Fittipaldi-Fernández et al.23. We can affirm that the results for weight loss are attributable to adequate regular multidisciplinary follow-up and the participants’ motivation. Maintaining multidisciplinary management with nutrition and workout measures is vital to avoid weight regain after device removal.
In our study, when making the comparative analysis by sex, a higher percentage of weight loss was found in women. These findings are consistent with earlier comparative studies in which a more significant loss of excessive weight has also been noted in women23,24. However, this has been attributed to the lower basal excess weight in women24, which may also be because, in our study, most of the population was women (75.22% of the cases). It is known that women are more willing to report gastrointestinal symptoms, request timely medical attention, receive recommendations for diagnostic tests, and adhere to treatment25. This study validates that women can receive prompt treatment for low- and moderate-risk obesity and achieve weight control goals in an optimal time. Further research is needed to clarify these findings.
Multiple studies have shown that 80% to 90% of weight loss is achieved during the first three to four months of IGB therapy; after this, the stomach accommodates, and the restrictive effect is partially lost, so an increase in the volume of the IGB is required to induce more significant weight loss17. The present study, evaluated the latest generation IGBs, which are adjustable; in other words, after three months of insertion, the weight loss effect is lost, so it is necessary to add volume to the IGB to change its volume and weight and achieve better results26.
Recent studies have corroborated the efficacy of the IGB brands used in this research. A meta-analysis in 2015, which included 17 studies with 1,638 patients, revealed an excess weight loss percentage of 25.44% (95% confidence interval [CI]: 21.47%-29.41%) and 11.27% total body weight loss at 12 months with Orbera IGB, which is considered an appropriate treatment option because it exceeded the intragastric preservation threshold and 5% total loss of body weight27. On the other hand, Schwaab et al. published a crossover study in 2020 in which 470 overweight and obese subjects were included, 144 of whom had a Spatz IGB implanted for up to 12 months, achieving total body weight loss of 15.5 ± 9.6%28. Regarding Elipse IGB, a meta-analysis by Ramai et al. examined seven studies with 2,152 patients and demonstrated similar results, with a total weight loss percentage of 12.2% (95% CI: 10.1-14.3, inconsistency index [I2]: 94%) and excessive weight loss percentage of 49.1% (95% CI: 30.6-67.5; I2 = 97%)29. Our study evaluated long-acting IGBs with a capacity of up to 12 months in the stomach and demonstrated better results and usefulness, as they allow more time for education on lifestyle changes, while short-acting balloons (less than four months) did not achieve significant weight loss. Although Orbera IGB was employed in more than 90% of the cases, corroborating its efficacy in therapeutic goals, the results were similar in Spatz and Elipse.
Limitations of this study include that it was a single-center study, and Orbera IGB was used in more than 90% of the subjects, preventing generalizability. Nevertheless, we could show the efficacy and complications of fluid-filled IGB in the adult population with low- and moderate-risk obesity, of which there is little literature in Colombia. It should be mentioned that only subjects older than 18 years were included, which limits its applicability to younger groups; however, the adult population included in the study is considered representative. The anthropometric evaluation of the patients was limited to bioimpedance. Other measures that could have added detail to the assessment of body changes and the impact of the IGB on body composition were not used. When evaluating the efficacy, other metabolic parameters such as glycosylated hemoglobin levels, lipid profile, and cardiovascular outcomes, which are of interest for this particular population, were not included. For being a retrospective study, the quality of the information may be affected when completing the medical records. Verification of clinical record data by at least two researchers could also decrease transcription bias.
Conclusions
The fluid-filled IGB is an attractive option for managing low- and moderate-risk obesity; it is a safe and effective procedure for achieving optimal weight loss goals.
Careful follow-up of the patient is paramount to avoid complications and support the efficacy of treatment; an IGB implantation period of at least 12 months is considered best for low- and moderate-risk obesity.
Since the IGB is a non-surgical and non-pharmacological temporary alternative for obesity, entirely reversible and repeatable, it should be especially recommended to patients with therapeutic failure of traditional weight reduction methods.











 text in
text in 



