Introduction
Esophageal achalasia is a rare entity with an incidence of one and a prevalence of ten per 100,000 inhabitants in the United States1. It is mainly characterized by dysphagia and chest pain but has other associated symptoms such as regurgitation and weight loss. The pathophysiological mechanism is not clearly understood, although the symptoms are attributed to the absence of peristalsis of the esophageal body and the lack of relaxation of the lower esophageal sphincter (LES)2. Thus, the term achalasia comes from the prefix a- and the Greek word khalasis, meaning “no relaxation.”
A high index of suspicion is required for the diagnosis since up to 40% of patients with achalasia may have a normal esophagogastroduodenoscopy (EGD)3. Contrast-enhanced radiography of the esophagus (esophagogram) classically shows dilatation of the esophagus and stricture at the esophagogastric junction with the “bird’s beak” ending sign4. However, the esophagogram can have up to 30% false negatives. In advanced disease, a severely dilated, angulated, and tortuous esophagus may be found and even have a sigmoid shape (megaesophagus)5.
Esophageal manometry confirms the diagnosis4. In clinical practice, manometry has two techniques: conventional and high-resolution (HR)6, the latter being more precise and allowing the achalasia type to be identified (I, II, or III), which can define the prognosis during treatment7,8. It has been concluded that type II responds better to any treatment, and type III predicts an adverse response9.
International guidelines and expert articles have proposed logical treatment algorithms10,11. Pharmacological therapy with nitrates and calcium antagonists is the least effective option and is reserved for patients who, due to their clinical condition, are not candidates for invasive therapies2. Other management options include endoscopic botulinum toxin injection and endoscopic pneumatic dilation. Botulinum toxin is applied to the LES, and its effectiveness for dysphagia control is 50% at one year12. Pneumatic balloon dilations of achalasia have early effectiveness similar to surgery, but their effect diminishes with time. Almost 50% of patients are estimated to require a new dilation in the 5-year follow-up13. Peroral endoscopic myotomy (POEM) is the most recent management strategy, which consists of performing an endoscopic esophagogastric myotomy with encouraging initial results14.
Surgical management with Heller myotomy is currently the standard for the definitive treatment of achalasia. Most studies show that surgery is more efficient than other alternatives, and, thanks to the laparoscopic approach, postoperative morbidity is very low15. In our setting, the clinical characteristics of patients with achalasia and the results of surgical treatment are unknown. This research aims to determine the degree of symptomatic improvement after laparoscopic Heller myotomy (LHM) and the morbidity of the procedure.
Materials and methods
Study type and patients
A retrospective descriptive study was carried out. Adult patients diagnosed with achalasia referred to LHM in two high-complexity healthcare facilities over eight years were studied. The information was obtained by reviewing medical records and telephone interviews with the patients.
Patients operated on via open surgery or from a thoracic approach, who could not be contacted by any means or with a previous myotomy were excluded. The variables explored were demographic and clinical characteristics, operative findings, postoperative complications, and follow-up.
The symptoms were assessed using the Eckardt symptom score, an instrument validated in the literature that includes the most relevant symptoms of patients with achalasia (dysphagia, retrosternal pain, regurgitation, and weight loss)16. The scale rates severity from 0 to 3, depending on the absence of the symptom (0) or its occasional (1), daily (2), or constant (3) presence. The total score is from 0 to 12, classifying the disease into stages: 0-1: stage 0, 2-3: stage I, 4-6: stage II, and > 6: stage III. Post-intervention stages 0 and I are defined as remission17.
Procedure description
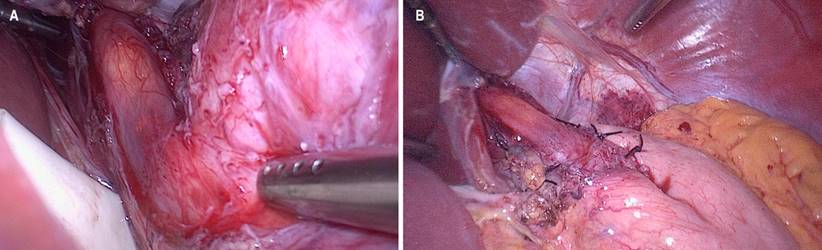
An American five-port surgical technique was used with the patient in the supine position with the operating bed tilted in reverse Trendelenburg. The dissection began on the left side, sectioning the short gastric vessels with an ultrasonic scalpel until the left crus of the diaphragm was identified. The pars flaccida is released, the left gastric artery and accessory hepatic artery are preserved, and if applicable, the phrenoesophageal membrane, both crurae, and the retroesophageal space are released. Once there is a circumferential dissection, an atraumatic traction of the esophagus is performed (with a Penrose drain), and its distal third is released at 360 degrees. The myotomy site is marked on the esophagus’s anterior side, respecting the vagus nerve’s left main trunk. The myotomy is then performed, which in most cases is performed bluntly with the help of two atraumatic forceps and, in others, with cautery. The length is, on average, 6 cm in the esophagus and 2 cm in the stomach. Intraoperative endoscopy is performed to verify the integrity of the mucosa, the length of the myotomy, and the complete opening of the cardia with insufflation of the endoscope (Figure 1A). The procedure is finished with a partial posterior (Toupet) or partial anterior (Dor) fundoplication at the surgeon’s discretion (Figure 1B). Drains are not used, and conventional port site closures are performed. The patient can start clear oral liquids the next day and continue with a blenderized diet for two weeks.
Ethical considerations
This study complied with the current regulations of healthcare institutions’ ethics and research committees and the research and bioethics committee of Universidad Antioquia. The confidentiality of the data obtained was guaranteed since only the researchers had access to the research instruments.
Statistical analysis
Continuous variables were described as means and ranges, while categorical variables were expressed as frequencies and proportions. For the comparison of continuous variables, a Student’s t-test was used. All statistical analyses were conducted with Stata v. 14 and GraphPad Prism 7.
Results
A total of 39 eligible patients were identified, of whom 11 were excluded due to insufficient information and one due to a reintervention; therefore, 27 patients were included in the analysis. 51% were men, and the average age was 48 years. 53.5% of the patients had comorbidities. Achalasia symptoms’ duration before surgery averaged 3.7 years (Table 1). All the patients underwent EGD, esophagogram, and esophageal manometry for diagnosis. Nine patients had AR manometry. Basal LES pressure had an average of 36 mm Hg. Three patients had prior endoscopic or medical treatment.
Operative time had an average of 133 minutes, the average length of the myotomy was 8.3 cm, and the mean bleeding was 34 mL (Table 2). Toupet-type fundoplication was performed in 25 patients. There were two intraoperative perforations of the esophageal mucosa that were repaired with separate 4-0 absorbable suture stitches, and in both cases, a Dor-type fundoplication was added. These two patients had a satisfactory postoperative evolution. In the intraoperative period, EGD was performed on 25 patients. The two mucosal perforations were confirmed by this means.
In the immediate postoperative period, 22 patients (88%) underwent an esophagram without reporting leaks or other complications. The average postoperative hospitalization was 2.7 days (1-14). A complication corresponding to infection associated with intravascular devices (phlebitis) occurred; this patient had a 14-day hospitalization for intravenous antibiotic treatment. There was no mortality in this series.
Table 1 Demographic and preoperative characteristics
| Variable | Frequency/Average | Percentage/Range |
|---|---|---|
| Sex | ||
| Female | 13 | 48.1% |
| Male | 14 | 51.8% |
| Age (years) | 48 | 18-76 |
| Cardiovascular disease | 8 | 29.6% |
| Lung disease | 2 | 7.4% |
| Metabolic disease | 3 | 11.1% |
| Duration of symptoms before surgery (years) | 3.7 | 1-20 |
| Achalasia type | ||
| Type I | 2 | 7.4% |
| Type II | 7 | 25.9% |
| Not reported | 18 | 66.6% |
| Basal LES pressure (mm Hg) | 36 | 20-58 |
LES: lower esophageal sphincter. Table prepared by the authors.
The Eckardt score was obtained in 21 patients. The average preoperative total score was 7.7 versus 1.2 postoperative (p < 0.001). Dysphagia had pre- and postoperative means of 2.5 and 0.2, respectively (p < 0.001), and improved in 95% of patients. Similar results occurred with chest pain and regurgitation. Weight loss improved in 100% of patients (p < 0.001) (Figure 2). Regarding the severity of the disease, it was found that in the preoperative period, six patients were in stage II (28.5%) and 15 in stage III (71.4%). After the intervention, 13 patients progressed to stage 0 (61.9%) and eight to stage I (38%).
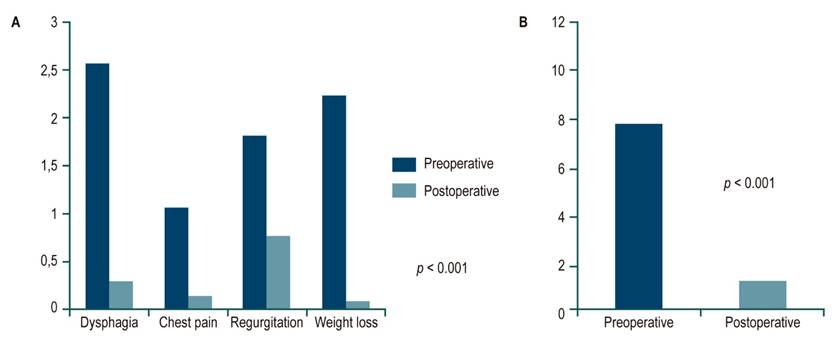
Figure 2 Comparison of symptoms by pre and postoperative Eckardt score. A. Comparison of averages by symptom type. B. Comparison of averages of the total score. p: Student’s t-test. Prepared by the authors.
The average postoperative follow-up was 24 months (1-87). A control esophagram and EGD were performed on six patients at the end of the year after the intervention. No other complications or recurrence were reported. Regarding gastroesophageal reflux (GER), five patients (18.5%) reported heartburn after surgery, and it was perceived as mild.
Table 2 Operative variables
| Variable | Frequency/Average | Percentage/Range |
|---|---|---|
| Surgical time (minutes) | 133 | 72-165 |
| Myotomy length (cm) | 8.3 | 6-10.5 |
| Intraoperative bleeding (mL) | 34 | 1-200 |
| Fundoplication type: | ||
| Toupet | 25 | 92.5% |
| Dor | 2 | 7.4% |
| Mucosal perforation | 2 | 7.4% |
| Intraoperative EGD | 24 | 88.8% |
EGD: esophagogastroduodenoscopy. Table prepared by the authors.
Discussion
Local articles on achalasia have been published in the last decade; however, there needs to be precise information on the results of surgical management in our population5,18,19. The present study is the first to describe patient demographics, procedure-related characteristics, and clinical outcomes of the intervention at medium-term follow-up.
The patients had a distribution according to sex and age similar to that in the literature20,21, with a shorter duration of symptoms than reported22. The frequency of symptoms was similar to other studies, and dysphagia was the main symptom21,23,24.
The length of the myotomy was very similar to other reports, with ranges of 6-8 cm25,26. We believe that a 6 cm myotomy in the esophagus and 2 cm in the stomach is sufficient to relieve the obstruction without increasing the risk of mucosal perforation or bleeding. Mucosal perforation occurred in two patients (7%), a finding within the reported rate (6.9%-7.8%)27-29. Fortunately, patients with mucosal perforations detected and repaired in the same surgical act have a similar postoperative course to others, as observed in the present study. Intraoperative EGD was performed on 88% of patients to assess the myotomy and confirm mucosal integrity more objectively. According to the experts’ recommendations, this intraoperative study is vital to guarantee better clinical results30.
It is estimated that the incidence of GER is up to 47.6% in patients with Heller surgery without fundoplication compared to 9.1% when a fundoplication is added31. In this study, 92.5% underwent a Toupet fundoplication, and the remaining 7.4% had a Dor type. The precise recommendation is that the fundoplication should be partial since there is a greater risk of dysphagia with the complete one (Nissen type). Although there are no significant differences in performing an anterior partial (Dor) or a posterior partial (Toupet)32,33, we are more inclined towards the Toupet-type fundoplication, which somewhat keeps the edges of the myotomy open and could prevent the formation of previous fibrosis and, consequently, the recurrence of dysphagia. We reserve the Dor type fundoplication for patients with perforation of the mucosa and in whom it is more logical to cover the suture line with the gastric fundus.
The surgeon’s experience determined surgical time and was similar to other studies25,26,31. Hospitalization time was shorter than that mentioned in other reports26,31. In this study, only one postoperative complication of a medical nature not directly related to the procedure occurred. The series had no mortality, confirming that LHM in expert hands is a highly safe procedure associated with almost zero morbidity.
There is substantial evidence demonstrating the effectiveness of LHM. Four meta-analyses showed that surgical treatment is the most appropriate management for patients with achalasia since it is associated with 100% resolution of dysphagia in one year and 77% in five years on average15,28,34,35. In this series, the improvement in dysphagia was 95% with an average follow-up of 24 months, in addition to the fact that 100% of the patients ended up in stage 0 or I in the Eckardt score, placing them in the disease remission category.
POEM is a recent therapeutic alternative mainly available in international reference endoscopy centers36. An initial experience in four patients with good results was published in our country37. The effectiveness of this technique could be comparable to LHM, but there still needs to be robust information or long-term follow-up38. Its main disadvantages are that it requires general anesthesia and specialized endoscopic instruments, the long learning curve, and the impossibility of adding an antireflux procedure, which could result in de novo GER in up to 40% of patients34.
Some limitations were identified in the study. First, control manometry was not performed to assess the impact of the intervention on LES pressure. This may be due to limitations inherent to our health system or the perception of the patient who considers further studies unnecessary after their symptoms improve. Still, it has been shown that Eckardt score values < 4 or stages < I correlate with manometry or esophagograms that show adequate esophageal function39. Since there is currently no clear recommendation on routine control studies such as EGD, esophagogram, manometry, or pHmetry, these should be performed depending on the patient’s symptoms.
Another significant limitation is its retrospective nature and the low number of patients, mainly due to the low prevalence of the disease. Likewise, some patients were lost to follow-up, which reduced the information available for analysis. However, this study may be the starting point for new research that makes a more objective and complete evaluation of LHM results through esophageal anatomy and physiology studies. This would make it possible to measure essential changes in the evolution of the disease, such as the diameter of the esophagus, the pressure of the LES, and exposure to acid reflux, among others.
Conclusion
Laparoscopic Heller myotomy is an effective and safe procedure for treating achalasia. This modality remains the management standard, resulting in a medium-term improvement of dysphagia in 95% of cases and a global symptomatic improvement in all patients. It is associated with a short hospital stay and minimal morbidity.











 texto en
texto en