Introduction
Autoimmune hepatitis is an inflammatory disorder of the liver that is likely to affect young and middle-aged women at a ratio of 2.4:0.9 compared to men1. It is characterized by histological and serological changes and the presence of autoantibodies in some patients2. Aminotransferases (formerly called transaminases) are located in hepatocytes and, when elevated, are sensitive indicators of hepatocyte injury3. On serum electrophoresis, approximately 80% of patients are expected to show hypergammaglobulinemia4. Antinuclear antibodies, smooth muscle antibodies, and liver and kidney microsomal antibodies may be present5. A liver biopsy is recommended unless the patient has contraindications, and the typical findings are interface hepatitis, rosettes, and plasma cell infiltration6.
The accentuated liver inflammation may result in cirrhosis by activating stellate cells responsible for producing fibrotic tissue, as they are the primary source of liver myofibroblasts. The fibrosis process can be triggered by the persistent presence of inflammatory cells such as infiltrating macrophages, hepatic macrophages (Kupffer cells), lymphocytes, and neutrophils7. Clinically, the challenge is finding markers that can be both sensitive and specific in predicting significant liver disease from the standpoint of inflammation and fibrosis.
This study aims to identify clinical features associated with age 50 or older and significant inflammation in liver histology.
Materials and methods
This analytical cross-sectional study evaluated medical records of adult patients with autoimmune hepatitis treated at the Gastroenterology and Hepatology Service of a tertiary referral University Hospital between January 2015 and December 2017. Patients with insufficient clinical and laboratory data in the medical records and patients who refused to participate were excluded.
The included individuals were analyzed regarding clinical, laboratory, and histological characteristics and their therapeutic response. Revised international criteria of the International Autoimmune Hepatitis Group were used to diagnose autoimmune hepatitis8. Data were collected from medical records and transferred to the Statistical Package for the Social Sciences (SPSS), v. 17.0 (Chicago, Illinois, United States). This study considered the following variables: age, sex, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), γ-glutamyl transferase (GGT), direct bilirubin (DB), serum albumin, prothrombin time (PT), antinuclear antibody, anti-smooth muscle antibody, and liver and kidney microsomal type 1antibodies (Anti-LKM1). ALT and AST were analyzed by the Clinical Chemistry System Dimension®, with ALTI Flex® and AST Flex® reagents at 37 oC. AP, GGT, DB, and serum albumin were also analyzed by the Clinical Chemistry System Dimension® at 37 oC. The reagent used for ALP was ALPI Flex®, and for DB, DBI Flex®. The PT was analyzed with a RecombiPlastin 2G® kit. All liver biochemical tests (AST, ALT, ALP, and GGT) were expressed in absolute numbers and three times the upper limit of normality (3 x ULN).
Regarding liver biopsy, the Brazilian Society of Pathology and Hepatology histological classification was used9. The following histological characteristics were observed: significant fibrosis (F ≥ 2), defined as a fibrotic portal expansion with portoportal septa or portocentral fibrotic septa or complete nodules; important portal inflammatory infiltrate (PII ≥ 2), defined as a sharp or very sharp increase in the number of portal lymphocytes; significant periportal inflammatory activity (PPA ≥ 2), defined as interface hepatitis or fragmentary necrosis, which may be discrete or present in extensive areas of numerous portal areas; and significant parenchymal inflammatory activity (PA ≥ 2), defined as focal necrosis of hepatocytes, surrounded by lymphohistiocytic aggregates at innumerable sites, with or without confluent necrosis, which may be extensive or multiple. Similarly, significant inflammation was defined as considerable inflammatory infiltrate, significant periportal inflammatory activity, and significant parenchymal inflammatory activity.
Statistical analysis
Continuous variables were described with central tendency and dispersion measures, while categorical variables were expressed in absolute numbers and proportions. Continuous variables were compared using Student’s t or Mann-Whitney U test, and categorical variables using chi-square or Fisher’s exact test when appropriate. A bivariate analysis was performed to identify characteristics associated with age equal to or greater than 50 and significant histological inflammatory activity. Spearman’s correlation analysis was performed to determine whether biochemical and liver function tests were correlated with age. P values less than 0.05 were considered statistically significant. All the tests were biflow and ran by SPSS, v. 17.0 (SPSS; Chicago, Illinois, United States).
This study protocol conforms to the ethical recommendations of the Declaration of Helsinki of 1975 and was approved by the university’s ethics and human research committee, number 1,147,617.
Results
Case study analysis
Fifty-eight patients with autoimmune hepatitis were evaluated in the study period to decide whether they would be included. Eleven were excluded due to insufficient clinical and laboratory data. Forty-seven patients were included; their mean, standard deviation and median age were 42.8 ± 16.0 (43.0) years, and 80.9% were women.
Regarding liver biochemistry (Table 1), the individuals had the following means, standard deviations, and medians: AST: 428.1 ± 475.5 (175.0) U/L; ALT: 372.1 ± 355.9 (250.5) U/L; ALP: 209.4 ± 122.0 (185.0) U/L; GGT: 237.7 ± 256.1 (180.0) U/L; DB: 3.6 ± 4.6 (1.2) mg/dL; blood pressure (BP): 57.6 ± 21.9 (60.3); albumin 3.4 ± 0.9 (3.5) g/L; γ-globulins: 2.8 ± 4.0 (1.8) g/L.
Table 1 Clinical and laboratory features of 47 patients with autoimmune hepatitis
| Clinical features | % | Mean ± SD | Median |
|---|---|---|---|
| Female | 80,9 | ||
| Age | 42,8 ± 16,0 | 43,0 | |
| AST (U/L) | 428,1 ± 475,5 | 175,0 | |
| ALT (U/L) | 372,1 ± 355,9 | 250,5 | |
| ALP (U/L) | 209,4 ± 122,0 | 185,0 | |
| GGT (U/L) | 237,7 ± 256,1 | 180,0 | |
| DB (mg/dL) | 3,6 ± 4,6 | 1,2 | |
| PA (%) | 57,6 ± 21,9 | 60,3 | |
| Albumin (g/L) | 3,4 ± 0,9 | 3,5 | |
| γ-globulins (g/L) | 2,8 ± 4,0 | 1,8 | |
| Histological features (n = 31) | |||
| Significant fibrosis | 64,5 | ||
| Significant periportal activity | 60,0 | ||
| Significant parenchymal activity | 33,3 | ||
| Significant portal inflammatory infiltrate | 27,3 | ||
| Rosettes | 18,5 | ||
| Plasma cell infiltrate | 62,0 | ||
AST: aspartate aminotransferase; ALT: alanine aminotransferase; ALP: alkaline phosphatase; DB: direct bilirubin; GGT: γ-glutamyl transpeptidase; PA: prothrombin activity. Source: The authors.
Regarding the antibodies, 58.7% featured a positive antinuclear antibody with titers ranging between 1:80 and 1:2560, 78.3% with a speckled pattern, 17.4% with a homogeneous pattern, and 4.3% with a filamentous pattern. Anti-smooth muscle antibody was positive in 52.2%, and 7.3% had positive anti-LKM-1. A few patients had more than one active antibody. Its distribution is shown in Figure 1.
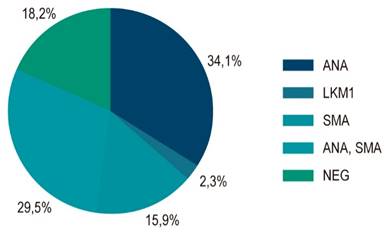
Figure 1 Distribution of the autoantibody profile in 58 patients with autoimmune hepatitis. ANA: antinuclear antibody; LKM-1: liver and kidney antimicrosomal antibody; SMA: anti-smooth muscle antibody; NEG: negative. Source: The authors.
Thirty-one individuals underwent liver biopsy, and ten (32.3%) presented with cirrhosis. Significant fibrosis (F ≥ 2) was found in 64.5%, as well as significant periportal activity (PPA ≥ 2) in 60.0% of the patients. 33.3% had significant parenchymal activity (PA ≥ 2), and 27.3% had significant portal inflammatory infiltrate (PII ≥ 2). Plasmocytes were found in 62% and hepatic rosettes in 18.5% of the patients.
Features associated with age equal to or greater than 50
When compared in terms of age (Table 2), individuals aged 50 or older had higher median GGTs (129 vs. 282 U/L; p = 0.034), and a higher proportion of individuals had GGT levels equal to or greater than three times the UPN (92.3% vs. 50%; p = 0.013). Regarding the histological findings of liver biopsies, patients aged 50 years or older showed a higher proportion of significant periportal inflammatory activity (83.3% vs. 38.5%; p = 0.041), significant parenchymal activity (58.3% vs. 13.3%; p = 0.037), significant portal inflammatory infiltrate (50.0% vs. 8.3%; p = 0.029) and, therefore, a higher proportion of significant inflammation (50% vs. to 6.7%, p = 0.024).
Table 2 Clinical and laboratory features associated with age over 50 of 47 patients with autoimmune hepatitis
| Features | < 50 years n = 32 | ≥ 50 years n = 15 | p† |
|---|---|---|---|
| Female (%) | 81,3 | 80,0 | 1,000 |
| AST (U/L)* | 464,9 ± 505,4 (230) | 346,2 ± 407,2 (128) | 0,461 |
| AST 3 x ULN (%) | 62,1 | 69,2 | 0,739 |
| ALT (U/L)* | 422,9 ± 410,5 (265) | 267,1 ± 168,5 (211) | 0,076 |
| ALT 3 x ULN (%) | 71,0 | 73,3 | 1,000 |
| ALP (U/L)* | 203,8 ± 136,5 (179) | 218,5 ± 97,6 (219) | 0,287 |
| ALP 3 x ULN (%) | 13,0 | 7,1 | 1,000 |
| GGT (U/L)* | 220,9 ± 294,5 (129) | 271,4 ± 158,8 (282) | 0,034 |
| GGT 3 x ULN (%) | 50,0 | 92,3 | 0,013 |
| DB (mg/dL)* | 3,6 ± 4,1 (1,7) | 3,6 ± 5,8 (1,2) | 0,784 |
| PA (%)* | 52,7 ± 27,2 (54) | 64,3 ± 9 (66) | 0,142 |
| Albumin (g/L)* | 3,5 ± 1,0 (3,5) | 3,4 ± 0,8 (3,6) | 0,795 |
| γ-globulins (g/L)* | 2,0 ± 0,9 (1,8) | 4,3 ± 6,7 (2,0) | 0,289 |
| Non-organ specific autoantibodies | |||
| ANA (%) | 51,6 | 73,3 | 0,161 |
| ANA titers ≥ 1:320 (%) | 50,0 | 54,5 | 0,816 |
| SMA (%) | 48,4 | 60,0 | 0,460 |
| LKM-1 (%) | 7,1 | 7,7 | 1,000 |
| Histological features (n = 31) | |||
| Significant fibrosis | 61,1 | 69,2 | 0,718 |
| Significant periportal activity | 38,5 | 83,3 | 0,041 |
| Significant parenchymal activity | 13,3 | 58,3 | 0,037 |
| Significant portal inflammatory infiltrate | 8,3 | 50,0 | 0,029 |
*mean ± standard deviation (median).† Pearson’s chi-square test, Fisher’s exact test, t-test, or Mann-Whitney U test, when applicable. 3 x ULN: three times the upper limit of normality; ALT: alanine aminotransferase; ALP: alkaline phosphatase; ANA: antinuclear antibody; AST: aspartate aminotransferase; GGT: γ-glutamyl transpeptidase; DB: direct bilirubin; LKM-1: liver-kidney microsome type 1; PA: prothrombin activity; SMA: smooth muscle antibody. Source: The authors.
Using Spearman’s rank order correlation method, no relationship was found between age and biochemical (ALT, AST, FA, GGT) or function (BD, ALP, and albumin) test values.
Features associated with significant inflammation
When comparing individuals with significant inflammation in the liver biopsy and individuals with mild inflammation (Table 3), the first group had a higher mean age (63.7 ± 14.0 vs. 41.0 ± 14.4; p = 0.001), with a higher proportion of patients equal to or older than 50 years (85.7% vs. 66.7%; p = 0.024). There was no significant difference between the two groups regarding sex, biochemical tests, function tests, γ-globulins, autoantibodies, and significant fibrosis.
Table 3 Clinical and laboratory features associated with significant inflammation in 31 biopsied patients with autoimmune hepatitis
| Features | Little inflammation n = 22 | Significant inflammation n = 9 | p† |
|---|---|---|---|
| Female (%) | 85,0 | 71,4 | 0,580 |
| Age (years)* | 41,0 ± 14,4 (40,0) | 63,7 ± 14,0 (59,0) | 0,001 |
| ≥ 50 years (%) | 30,0 | 85,7 | 0,024 |
| AST (U/L)* | 565,2 ± 554,9 (293,5) | 494,0 ± 543,8 (210,5) | 0,787 |
| AST 3 x ULN (%) | 66,7 | 83,3 | 0,629 |
| ALT (U/L)* | 427,3 ± 419,5 (307,5) | 388,3 ± 265,4 (439) | 0,821 |
| ALT 3 x ULN (%) | 75,0 | 85,7 | 1,000 |
| ALP (U/L)* | 182,7 ± 102,1 (179) | 263,9 ± 98,5 (235) | 0,095 |
| ALP 3 x ULN (%) | 6,7 | 14,3 | 1,000 |
| GGT (U/L)* | 189,4 ± 150,3 (155) | 292,1 ± 200,1 (293) | 0,193 |
| GGT 3 x ULN (%) | 60,0 | 85,7 | 0,350 |
| DB (mg/dL)* | 4,4 ± 5,7 (1,9) | 3,9 ± 3,2 (0,8) | 0,526 |
| PA (%)* | 57,7 ± 19,9 (59) | 54,7 ± 28,8 (64) | 0,818 |
| Albumin (g/L)* | 3,6 ± 0,9 (3,6) | 3,0 ± 0,9 (2,9) | 0,241 |
| γ-globulins (g/L)* | 2,0 ± 1,0 (1,7) | 2,6 ± 1,2 (2,6) | 0,280 |
| Non-organ specific autoantibodies | |||
| ANA (%) | 50,0 | 71,4 | 0,408 |
| ANA titers ≥ 1:320 (%) | 80,0 | 40,0 | 0,251 |
| SMA (%) | 40,0 | 85,7 | 0,077 |
| LKM-1 (%) | 5,6 | 0,0 | 1,000 |
| Histological features | |||
| Significant fibrosis | 52,6 | 66,7 | 0,661 |
*mean ± standard deviation (median).† Pearson’s chi-square test, Fisher’s exact test, t-test, or Mann-Whitney U test, when applicable. 3 x ULN: three times the upper limit of normal; ALT: alanine aminotransferase; ALP: alkaline phosphatase; ANA: antinuclear antibody; AST: aspartate aminotransferase; GGT: γ-glutamyl transpeptidase; DB: direct bilirubin; LKM-1: liver-kidney microsome type 1; PA: prothrombin activity; SMA: smooth muscle antibody. Source: The authors.
Discussion
GGT is a well-known, established serum marker for steatosis and alcohol-related diseases. When associated with elevated alkaline phosphatase levels, it indicates cholestasis, highly suggestive of intra- or extrahepatic biliary injury. GGT, found in hepatocytes and biliary epithelial cells, is a sensitive marker for biliary tract diseases such as cholestasis, but not very specific10. It was also elevated in extrahepatic conditions such as acute coronary syndrome, renal failure, diabetes, dementia, and pancreatic disease. However, in these conditions, GGT is more likely to have increased due to oxidative stress caused by changes in homeostasis, thus leading to cell destruction, damage, and death11,12. Some drugs can also increase the levels of GGT in the blood13.
Previous studies have shown a significant association between GGT levels and AST in patients with hepatitis C14-16. Another study has shown that a substantial number of patients with chronic hepatitis C virus (HCV) infection had elevated levels of serum GGT and a more intense level of necroinflammatory activity in patients with higher GGT. Thus, this enzyme has been proposed as a surrogate marker of significant inflammation in chronic hepatitis C17. Although these are remarkable results, no studies indicate the role of GGT in the level of inflammation and fibrosis/chronic disease in patients diagnosed with autoimmune hepatitis.
A retrospective study of 23,597 healthy individuals found that the medians and interquartile ratios were higher in the very young compared with those 60 years of age or older (27.1 [18.8-41.7] vs. 22.5 [16.3-32.7] U/L, p < 0.001)18. This finding suggests that the observation of higher GGT levels in older patients in our study reflects the higher inflammatory activity found in this group, not age itself.
Autoimmune hepatitis is generally characterized by a bimodal age pattern at the onset, with a peak in children and adolescents and a second in midlife (fourth to sixth decades and especially in postmenopausal women). However, a considerably increasing number of patients are even older than 65-70 years19. Few studies have evaluated the clinical features of autoimmune hepatitis in older adults. In the Italian elderly, autoimmune hepatitis is usually asymptomatic, although the prognosis and response to treatment are like those of younger patients. However, no difference was observed in liver disease’s histological/biochemical expression20. Onset at an early age, acute manifestation, hyperbilirubinemia, and the presence of HLA DRB1*03 characterize patients who fail corticosteroid treatment21. A North American study assessed 205 adults with defined autoimmune hepatitis type 1 and grouped them according to age of manifestation. Twenty-three percent of the patients were ≥ 60, and 15% were ≤ 30. Patients ≥ 60 years had a higher frequency of cirrhosis at onset than patients ≤ 30 years (33% vs. 10%, p = 0.03) and also failed corticosteroid treatment less frequently than patients ≤ 30 years (5% vs. 24%, p = 0.03)22.
In China, elderly autoimmune patients have a higher frequency of cirrhosis at onset and a lower occurrence of treatment failure. Older patients had similar mean GGT levels (112.8 ± 82.8 vs. 121.9 ± 103.2 U/L; p = 0.146), and histological features were not evaluated23. In the United Kingdom, 164 patients with autoimmune liver disease were evaluated. When individuals aged 40 years or older were compared with those younger than 40 years, similar mean GGT levels (103.5 [8-820] vs. 190 [29-995 ] U/L; p = 0.040) and similar levels of histological grade of necroinflammatory activity (2 vs. 2 [mild]; p = 0.022), different data were observed in older patients24 than those in the present study. A systematic review and meta-analysis demonstrated that GGT levels do not differ when comparing the elderly with the young (30 vs. 27 U/L; p = 0.039), and histological features were not examined25.
A liver biopsy is necessary to diagnose autoimmune hepatitis, and establishing this diagnosis without histology should be an exception and limited to special clinical situations26. Portal lymphoplasmacytic hepatitis with interface activity and lobular inflammation is frequently found in autoimmune hepatitis26,27. Recently, severe necroinflammatory activity in autoimmune hepatitis has been associated with a serum level of 25(OH)D28. Unfortunately, vitamin D levels were not available in this study. When patients with hepatitis C were studied, severe interface hepatitis was associated with epidemiological features such as older age at both infection and biopsy and a higher prevalence of blood transfusion and alcohol abuse29. Also, in patients with hepatitis C, age > 40 years and the degree of inflammatory activity were associated with elevated levels of GGT17.
In conclusion, individuals 50 or older had higher median GGT and a higher proportion of significant inflammation on liver histology. Moreover, considerable inflammation on liver biopsy was associated with advanced age.











 texto en
texto en 



