Introduction
Tuberculosis (TB) is a preventable and curable infectious disease associated with high morbidity and mortality worldwide; it was declared by the World Health Organization (WHO) in 1993 as a public health emergency1,2. Its primary clinical compromise is the lung, known as pulmonary TB; however, it can occur in other organs such as the pleura, lymph nodes, abdomen, genitourinary tract, and joints, among others, and together it is called extrapulmonary TB3. By 2020, 9.9 million cases of TB had been documented, with an estimated incidence of 127 cases per 100,000 people and an approximate mortality of 1.2 million people per year in patients without human immunodeficiency virus (HIV) coinfection and 214,000 people per year in patients with HIV coinfection4. It has been reported that patients with multiple comorbidities may be affected by TB infection5. Nonetheless, there are few reports on patients with underlying congenital disorders. We present the case of a 31-year-old patient with a history of thalassemia. He was admitted due to abdominal pain and constitutional symptoms, in whom neoplastic involvement was suspected upon initial approach, and intestinal TB was documented during the diagnostic process. The studies conducted for the characterization of the disease are provided.
Case presentation
A 31-year-old male patient who worked as a farmer presented with a clinical picture of two years of evolution consisting of intermittent colicky abdominal pain in the right hypochondrium of moderate intensity and 4-5 stools a day of Bristol consistency 6, without mucus or blood. He reported the association with asthenia, adynamia, quantified weight loss of seven kilograms in the last six weeks, and nocturnal diaphoresis for two weeks. He had an increase in the intensity of abdominal pain in the previous five days associated with proctalgia and hematochezia, so he decided to attend the emergency room at our institution. On review by systems, he had respiratory symptoms that began eight days before admission due to dry cough and mMRC (modified Medical Research Council) dyspnea 3/4, associated with the appearance of ulcerated lesions in the anal region. The patient had a history of β-thalassemia with hepatosplenomegaly, for which he received a folic acid replacement and blood transfusions as needed; he also had a history of hepatitis A infection and a family history of colon cancer in his mother, diagnosed at age 52. On admission, the patient had tachycardia, diffuse abdominal pain on palpation, and palpable hepatomegaly over the right costal margin. The lymph nodes in the proper inguinal chain were not attached to deep planes or tender on palpation. The anal inspection revealed an ulcerated lesion around 9:00 clockwise with regular edges and no bleeding.
A clinical history of gastrointestinal neoplasia was considered because of the family history. An abdominal tomography with contrast was requested, which identified a concentric thickening of the wall of the ascending colon with slight enhancement and multiple mesenteric and retroperitoneal adenopathies (Figure 1). The paraclinical tests requested on admission documented normochromic normocytic anemia, lymphopenia, mild thrombocytopenia, elevated alkaline phosphatase, and hypoalbuminemia.
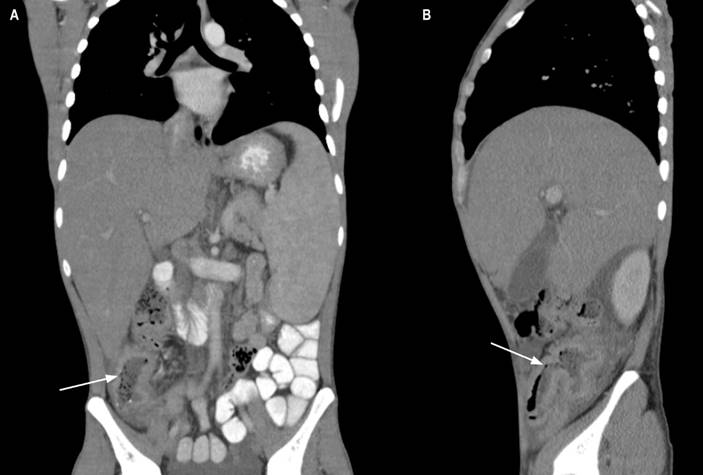
Figure 1 Contrast-enhanced coronal and sagittal computed tomography of the abdomen. A. Concentric thickening of the wall of the ascending colon with slight enhancement (arrow). B. Thickening of the distal ileum and ileocecal valve (arrow). Source: Patient’s medical record.
A colonoscopy was performed, which revealed multiple 5-mm ulcers with raised and defined erythematous edges at the level of the rectum, sigmoid, descending, and transverse colon, edematous mucosa covered by fibrin and erythema at the level of the hepatic flexure that diminishes friable lumen upon passage of the equipment, and an area of concentric stenosis with an inflammatory appearance. Colitis of infectious or inflammatory etiology was considered among the possible diagnoses. Biopsies of the mentioned areas were taken, documenting findings compatible with granulomatous colitis (Figure 2). A rapid polymerase chain reaction test was performed to identify Mycobacterium tuberculosis and sensitivity to rifampicin (GeneXpert MTB/ RIF) in the tissue obtained in the colon biopsy, with a positive result for M. tuberculosis (Table 1). Due to respiratory symptoms, a chest tomography was requested, which reported micronodules of bilateral random distribution, some forming a budding tree pattern and predominated in the left upper lobe (Figure 3). Serial bacilloscopies found acid-fast bacillus (AFB), and the GeneXpert MTB/RIF test confirmed the diagnosis of TB and lung involvement with sensitivity to rifampicin.
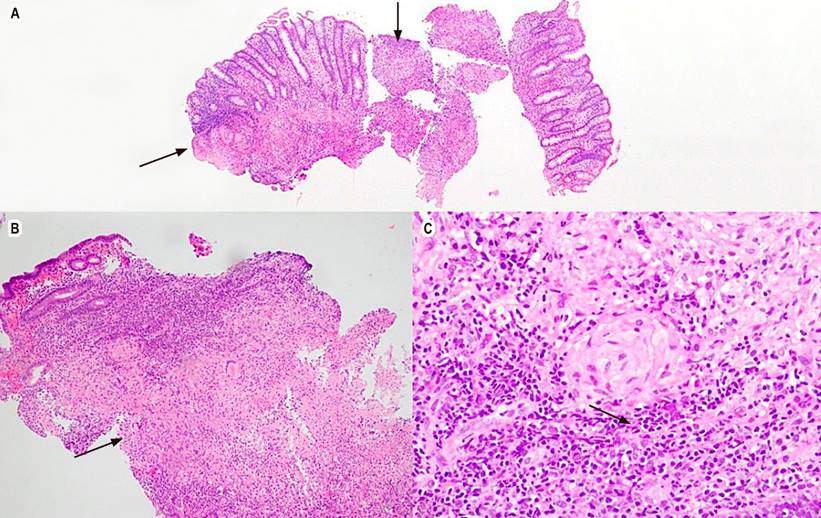
Figure 2 Granulomatous colitis with hematoxylin and eosin. A. 4x, colonic mucosa, arrows point to granulomas. B. 10x, the lamina propria shows the formation of well-defined granulomas and some neutrophil abscesses. C. 40x, epithelioid granuloma. Source: Patient’s medical record.
Table 1 Summary of tests and results of the patient
| Colonoscopy samples | |
|---|---|
| Transverse and right colon biopsy | Negative for malignancy Findings consistent with granulomatous colitis |
| PCR in rectal biopsy* | Sample positive for tuberculosis complex mycobacteria |
| Sputum | |
| Staining for AFB | Positive 1-9 AFB in 100 observed fields |
| PCR in sputum sample | Identification of M. tuberculosis by PCR: Detected Rifampicin resistance not detected |
| Lowenstein-Jensen culture | M. tuberculosis |
| MGITTM liquid culture medium | A positive culture for M. tuberculosis complex |
| Sensitivity tests*** | |
| Isoniazid | 0.1 µg/mL sensitive (S) |
| Rifampicin | 0.5 µg/mL sensitive (S) |
| Pyrazinamide | 100 µg/mL sensitive (S) |
| Peritoneal fluid | |
| Gram stain in ascitic fluid | No germs observed |
| Ascitic fluid culture | Negative at 72 hours of culture |
| Cytological | |
| Appearance | Limpid |
| Color | Yellow |
| Leukocytes | 234 cells/mm3 |
| Lymphocytes | 74% |
| Monocytes | 8% |
| Neutrophils | 18% |
| Macrophages | 118 in 100 leukocytes counted |
| Red blood cells | 786 cells/mm3 |
| Cytochemical | |
| Glucose | 98.19 mg/dL |
| Proteins | 3.38 g/dL |
| Albumin | 1.5 g/dL |
| Serum ascites albumin gradient | 1.1 |
| Microbiology | |
| Aerobic blood culture #2 | Negative after five days of culture |
| Anaerobic blood culture #1 | Negative after five days of culture |
| HIV test**** | 0.06 sec/CO; NOT reactive |
*Real-time PCR using TaqMan probes for the IS6110 repeat region of tuberculosis complex mycobacteria.
**Real-time, nested, semi-quantitative PCR and melt peak detection. DNA detection (IS1081-IS6110) of M. tuberculosis (MTB) and detection of rpoB gene mutations associated with resistance to rifampicin.
***BACTEC MGIT 960 automated method.
****Chemiluminescent Microparticle Immunoassay (CMIA).
Source: Prepared by the authors with data from the patient’s medical record.
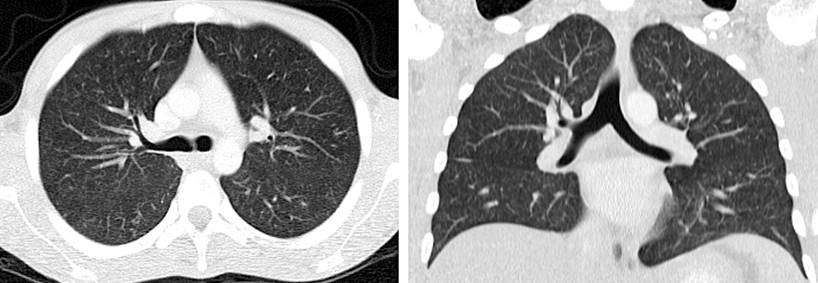
Figure 3 Coronal and cross-sectional high-resolution chest CT. Bilateral randomly distributed micronodules, some forming a budding tree pattern. Source: Patient’s medical record.
An ophthalmology opinion was requested for the initiation of tetraconjugate treatment. In the assessment, an incidental finding in the left eye revealed a lesion with a granulomatous subretinal appearance consistent with ophthalmic involvement of TB. He was considered a patient diagnosed with disseminated TB, pulmonary, intestinal, lymphatic, and retinal involvement with sensitivity tests that confirmed sensitivity to isoniazid and rifampicin. Thus, tetraconjugate management was started with rifampicin 150 mg, isoniazid 75 mg, pyrazinamide 400 mg, and ethambutol 275 mg, each dose adjusted for weight. The first phase consisted of 56 doses, and pyridoxine, one tablet per day, was added. The patient was discharged.
He was re-admitted to our institution 20 days after discharge due to a clinical picture compatible with intestinal obstruction, for which an abdominal X-ray was taken, finding staggered air-fluid levels and the absence of distal gas. Medical management with a nasogastric tube and analgesia was indicated, and an abdominal tomography was requested with a new finding of ascites. A paracentesis was ordered in which 200 mL of sallow fluid was obtained, and the cytological study found leukocytosis at the expense of lymphocytes. In addition, the serum ascites albumin gradient (SAAG) confirmed a nonhypertensive fluid (Table 1), which, due to its cytochemical and cytological characteristics and a history of disseminated TB, was deemed ascitic fluid consistent with peritoneal TB. The obstructive symptoms resolved, and the abdominal pain improved, for which the patient was discharged from the hospital.
Discussion
Disseminated TB is defined as TB infection in two or more non-contiguous sites resulting from lymphohematogenous dissemination of M. tuberculosis, which occurs due to a primary infection or reactivation of a latent focus6,7. The prevalence has been reported between 4.9% and 19.6% of patients diagnosed with TB, and patients with HIV coinfection have the highest probability of disseminated involvement (27.8%)8-10.
Among the independent risk factors described for the development of disseminated TB are the use of immunosuppressive drugs, coinfection with HIV, liver failure or cirrhosis, duration of symptoms ≥12 weeks, bilateral lung involvement, weight loss, nocturnal diaphoresis, and absence of hemoptysis and dyspnea10. The most frequent clinical manifestations include constitutional symptoms such as fever and nocturnal diaphoresis and abnormal laboratory findings such as hypoalbuminemia (74.7%), elevated γ-glutamyl transferase (71.0%), alkaline phosphatase (66.4%), hyponatremia (58.9%), and anemia (43.9%)11. Mortality from disseminated TB has been reported in around 21% of patients monitored for six months, and levels of albumin, total bilirubin, creatinine, and time to start anti-tuberculosis treatment have been correlated as independent prognostic factors10,11.
The thalassemias are a group of hemoglobinopathies in which the standard ratio of alpha globin to beta globin production is disrupted due to a genetic variant. This abnormal ratio of alpha to beta chains causes unpaired chains to precipitate, destroying red blood cell precursors in the bone marrow and leading to ineffective erythropoiesis and hemolysis12.
In many areas where TB is endemic, thalassemia is also high, especially in Southeast Asia13,14. Sriwijitalai et al.15 conducted a bioinformatic analysis of the biological pathways related to the antioxidant system in TB and thalassemia. They identified the pathway common to TB and thalassemia through glutathione, a pleiotropic antioxidant molecule showing antimycobacterial and immune-enhancing effects. The importance of the antioxidant pathway in TB has been demonstrated. Cao et al.16 observed a significant decrease in intracellular glutathione levels in macrophages infected with M. tuberculosis compared to uninfected macrophages, indicating that M. tuberculosis infection can cause depletion of intracellular glutathione and, in turn, promote the survival and replication of M. tuberculosis within host cells. Regarding thalassemia, the clinical importance of the antioxidant pathway system is highlighted. β-thalassemia is known to cause oxidative stress induced by iron overload, and the glutathione system is the primary endogenous antioxidant that protects animal cells from oxidative damage17.
Under oxidative stress, glutathione (GSH) donates reducing equivalents to free radical-scavenging enzymes, including glutathione peroxidase (GPx) and glutathione-S-transferase (GST). It is converted to its oxidized form, glutathione disulfide (GSSG). This GSSG can be reconverted to GSH by a reaction catalyzed by glutathione reductase (GR); therefore, a lower ratio of reduced to oxidized glutathione (GSH/GSSG) may indicate increased oxidative stress in cells. Kalpravidh et al.18 found a 90% reduction in the GSH/GSSG ratio in patients with β-thalassemia compared to controls, suggesting lower GSH availability and more significant GSSG accumulation. The marked increase in GSSG in thalassemia patients was probably due to overutilizing cellular GSH, supported by 123% and 93% increases in GST and GPx activities, respectively. The GST enzyme detoxifies xenobiotics, including metabolites from oxidative reactions, by conjugation with GSH. At the same time, GPx is an antioxidant enzyme that reduces hydrogen peroxide to water using GSH as the reducing equivalent.
It has been widely reported that patients with marked deficiency in GSH production had impaired granulomatous effector responses against M. tuberculosis. Patients with thalassemia are prone to developing TB, which is explained by the GSH pathway19. Additionally, the functions of iron overload and susceptibility to infection have been previously described20-24. However, publications on the thalassemia model have been limited. Most studies have used hemochromatosis as a disease model for iron overload.
Ghozali et al.25 reported on the types of variations of a metal transporter across the phagosome membrane, natural resistance-associated macrophage protein 1 (NRAMP1), in pediatric thalassemia patients with and without TB infection. They noted that NRAMP1 polymorphisms play an essential role in iron regulation, which is also necessary for MTB. Increased iron in patients with thalassemia may have a higher potential risk of TB26.
The clinical case we present has several highlights, the main one being the manifestation of disseminated TB in an immunocompetent patient with a history of β-thalassemia, which had not previously been considered a risk factor for this manifestation. However, in the literature review, we found biological plausibility in the alteration in the GSH pathway, which confers risk to patients with thalassemia of developing TB due to alteration in the granulomatous effector response. Another critical point to consider is the presence of previously described risk factors for developing the disease, which was identified in our patient as a manifestation of symptoms ≥ 12 weeks, constitutional symptoms due to weight loss and nocturnal diaphoresis, and bilateral lung involvement, which implies a progression of the local disease that can precede systemic involvement. Additionally, abnormal findings in our patient’s laboratory tests, such as hypoalbuminemia and anemia, have been correlated with mortality; however, during the follow-up of our patient, he only had one hospital readmission, and it was due to intestinal obstruction favored by the inflammatory process at the colonic level due to TB, which was resolved with medical management.
Cases described in the Colombian literature were reviewed. We found an extrapulmonary manifestation with compromise in the central nervous system27, pericardium with cardiac tamponade as the first manifestation28, sternum29 in the pediatric population, and a case of a patient with diabetes mellitus, and thalassemia and pulmonary TB infection with multiple associated coinfections30. To the best of our knowledge, the case presented is the first report of a patient with thalassemia and disseminated TB; it sets a relevant precedent for future research and the expansion of differential diagnoses in similar cases.
Conclusions
Disseminated TB in immunocompetent patients is a rare manifestation associated with adverse outcomes. Therefore, prompt diagnosis is required to reduce disease-associated morbidity and mortality. The present case allows us to conclude that there should be an active search and similar diagnostic suspicion in patients with a history of β-thalassemia, given their immunological predisposition. However, more studies are required to define screening strategies in immunocompromised patients.











 texto en
texto en 



