Introduction
Pancreatic collections are a complication of pancreatitis. More than half usually resolve spontaneously; the best decision is often expectant or conservative management1,2. The updated Atlanta classification3 describes that, in the late phase of pancreatitis, after more than four weeks, pancreatic pseudocysts develop from interstitial edematous pancreatitis or walled-off pancreatic necrosis resulting from necrotizing pancreatitis. Superinfected or symptomatic pseudocysts require interventional management, and current therapeutic modalities include surgical, endoscopic, or percutaneous drainage4.
In the last decade, there has been a paradigm shift in managing pancreatic collections secondary to pancreatitis, and drainage guided by endoscopic ultrasound (EUS) has become the technique of choice5,6. Recently, new generation ultrasound-guided stent systems with larger diameters have been described. These devices allow precise and adequate drainage of the collections and the performance of endoscopic necrosectomies through these stents7, called lumen-apposing metal stents (LAMS). Their implementation, safety, and effectiveness have been demonstrated for transmural drainage of pancreatic collections by endoscopic means, with tremendous therapeutic success, shorter hospital stays, and reduced general costs compared to surgical or percutaneous drainage8-10. Locally, Gómez et al.11 describe the use of conventional metal stents in the drainage of ten pancreatic collections. Still, no studies are related to the effectiveness and safety of using LAMS.
This study evaluated the effectiveness and safety of newly designed LAMS for EUS-guided drainage in treating symptomatic pancreatic pseudocysts in two referral centers in Colombia.
Materials and methods
Study design and data extraction
A multicenter prospective cohort study was conducted with convenience sampling. Thirteen patients with a diagnosis of symptomatic pancreatic pseudocysts treated between June 2019 and December 2021 in two gastroenterology referral centers in Bogotá, Colombia, were included. The study population consisted of adults (18 years or older) who developed symptomatic pancreatic pseudocysts defined by abdominal pain or gastric restrictive pattern (postprandial discomfort with a sensation of early fullness) with more than eight weeks of evolution since the documentation of pancreatitis. Eligible patients were required to have active clinical follow-up at each study institution. Subjects under 18 years of age, organized pancreatic necrosis (with a minimal fluid component), infected pseudocysts, hemodynamic instability, and severe coagulopathy (international normalized ratio [INR] >1.5 or platelet count <50 × 109/L), use of anticoagulation/antiplatelet therapy that cannot be discontinued, and refusal to give informed consent to participate in the study were the exclusion criteria.
Data collection
The medical records and the official report of the procedure performed were used as the primary source of information. Patients underwent EUS-guided drainage with a newly designed LAMS (Niti-S HOT SPAXUS; Taewoong Medical Co, Ltd, Ulsan, South Korea) with diameters of 16 mm and 10 mm and a length of 20 mm, according to stock availability. Outcome variables included technical success, clinical success, and successful stent removal. Patients were followed prospectively until stent removal.
Definitions
Technical success was defined as adequate release and positioning of the stent at both ends. Clinical success was described as a greater than 50% decrease in collection at four weeks, determined by follow-up imaging, EUS, or computed axial tomography (CT). Clinical failure was considered as the persistence of the collection or symptoms after completing four weeks of collection drainage. If the imaging follow-up did not show complete or expected collection drainage, the stent was maintained for an additional four weeks, and a new imaging check-up was performed in 3 to 6 months with EUS. No plastic stents were used. Successful stent removal was defined as removing the prosthesis without complications such as bleeding. Procedure time was defined as the time between puncture and stent deployment12,13. Safety measures included stent-related adverse events (AEs) and overall adverse events (OAEs).
Statistical analysis
The database was prepared in Excel version 2019. Missing data were filled in with new reviews of data sources, and only complete data analyses were performed at the end. The study’s primary endpoint was clinical success during stent removal. This single-arm study aimed to confirm its non-inferiority based on comparing the clinical success of the Niti-S HOT SPAXUS stent with reference values. The weighted average calculated in previous studies was around 96%, which was established as the expected clinical success for this study.
Data processing was carried out in the social science program SPSS version 25.0. For the descriptive analysis of the quantitative variables, we used the arithmetic average, the minimum, and the maximum, while for qualitative variables, absolute and relative frequencies.
Ethical considerations
The ethics and research committees of the respective institutions in Bogotá, Colombia, approved this study. Both are tertiary care hospitals and referral centers in gastroenterology. Its design contemplated the requirements in Resolution 8430/1993 issued by the Colombian Ministry of Health, so it was deemed a low-risk study, and the confidentiality of the information collected was guaranteed. All patients were instructed regarding the intervention and signed the informed consent. None of the records contained sensitive information about the identity of the patients.
Results
Thirteen patients underwent surgery (average age 53.4 years; eight were men), whose clinical characteristics are summarized in Table 1. The mean size of the pseudocyst was 9.56 ± 2.3 cm.
Table 1 Demographic, clinical, and procedural characteristics of patients
| Characteristics (n = 13) | ||
|---|---|---|
| Age, mean (SD) | 53.4 (8.1) | |
| Sex | ||
| Male, n (%) | 8 (61.5) | |
| Female, n (%) | 5 (38.5) | |
| Pseudocyst characteristics | ||
| Size, mean (SD) | 9.6 (2.3) | |
| Drainage to stomach, n (%) | 13 (100) | |
| Stent characteristics | ||
| Stent diameter | ||
| 15 mm, n (%) | 11 (84.6) | |
| 10 mm, n (%) | 2 (15.4) | |
| Procedure | ||
| Withdrawal time (weeks), mean (SD) | 8 (2) | |
| Procedure time (minutes), mean (SD) | 3.2 (2.4) | |
| Clinical outcomes | ||
| Technical success, n (%) | 13 (100) | |
| Clinical success, n (%) | 12 (92.3) | |
SD: standard deviation. n: number. Table prepared by the authors.
Technical aspects of placement
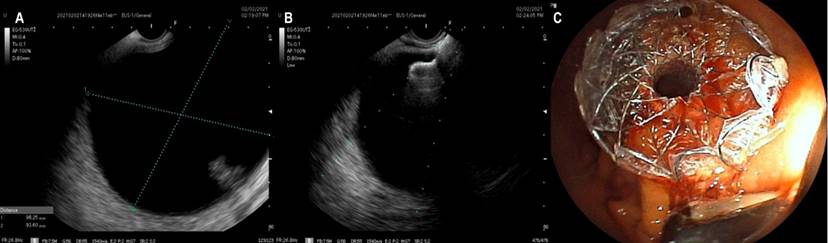
The patients underwent sedation assisted by anesthesiology using propofol; general anesthesia was not required. Ciprofloxacin 400 mg IV (single dose) was used as antibiotic prophylaxis. A Fujifilm EG-530UT2 linear echoendoscope with a SONART SU-1 ultrasound processor operated by an expert echo-endoscopist was used. Once the collection was evaluated by EUS (Figure 1A), a puncture was performed using a Doppler guide to check for the existence of interposed vessels.
All drainage was performed to the stomach. In only four cases, a 19 G puncture needle and guide wire of 0.035 were used under echoendoscopic vision to ensure the path was not lost. However, in the following cases, neither needle nor guide was used, taking into account better familiarization with the new stent and its ease of use, in addition to a good ultrasound window (Figure 1B) and size greater than 6 cm in diameter of the pseudocysts. An ERBE VIO 200S electrosurgical unit was used in the CUT mode at 200 W effect 6. We did not culture the samples obtained. In no case was an abscess or areas of walled-off necrosis documented. It is noteworthy that fluoroscopy was not employed, so there was no additional need for contrast media.
In none of the cases, we used dilation balloons. The radial expansion of these devices is adequate, so we consider that it is unnecessary for improving outcomes (Figure 1C). The most used diameter was 15 mm (eleven patients) and 10 mm (two patients); the decision to use these calibers was due to their availability in the institutions at the time.
Clinical outcomes
Technical success was 100% (13/13) and clinical success was 92.3% (12/13). The only case without clinical success was a patient who presented with bleeding 48 hours after the procedure due to a rupture of a splenic artery pseudoaneurysm when the cavity collapsed, which required surgical intervention with a good outcome. All stents were successfully removed using the foreign body clamp at 8 ± 2 weeks on average. The mean procedural time from puncture to stent deployment was 3.2 ± 2.4 minutes (Table 1). Imaging follow-up (CT, magnetic resonance imaging [MRI], or ultrasound endoscopy) at eight weeks allowed us to see adequate drainage of the collections (greater than 80%) in all cases. No differences were observed between the diameters of the stents used, possibly because they were only pseudocysts without areas of necrosis or a more significant component of associated debris.
Security profile
Regarding complications, in one case, bleeding was documented 48 hours after stent placement, with no transfusion requirement and the need for surgical intervention due to rupture of the splenic artery’s pseudoaneurysm when the pseudocyst cavity collapsed. Evolution was satisfactory.
Discussion
The technique of endoscopic drainage of pancreatic pseudocysts has evolved in recent years. Previously, multiple plastic stents were used to drain pancreatic pseudocysts, which were associated with migration, smaller diameters, and numerous access requirements. This resulted in the search for alternatives, such as metal stents, to achieve efficient drainage2. The implementation of EUS as a puncture guide also allows for defining the characteristics of the pancreatic collection, particularly in the absence of compression to neighboring organs, because it identifies the lesionable adjacent vasculature and rules out neoplasms14, which is associated with better safety and reduced complications10. This study shows that EUS-guided drainage using LAMS (Niti-S HOT SPAXUS; Taewoong Medical Co, Ltd, Ulsan, South Korea) is an excellent alternative because it reduces the comorbidities previously observed with other drainage techniques.
In our study, a technical success rate of 100% (13/13) was achieved, consistent with what is described in the literature15-17, and is likely related to the role of the operator’s experience. A clinical success of 92.3% was also found, which suggests that although all patients included in the study were symptomatic and adequate release and positioning of the stent was performed (12/13), a low percentage of the cases (7.7%) did not attain a reduction of more than 50% in the collection at four weeks. It could affect clinical practice in the future since symptomatology takes precedence over radiological findings. These findings are per the recommendations of the revised Atlanta classification3: the initial clinical intervention for a pancreatic collection should be guided by clinical symptoms and not by the size of the cyst. Besides, clinical signs should guide endoscopists for future interventions after stent placement.
Currently, endoscopic drainage of pancreatic pseudocyst is the preferred option in many referral centers, with reports of success greater than 93% and morbidity less than 10%, without mortality10,17. EUS drainage is associated with an increase in technical success and a decrease in the incidence of complications4. This study demonstrated technical success in 100% (13/13) of patients, with few complications related to the procedure (1/13 patients) and resolution of the condition greater than 90% (clinical success was 92.3%). Therefore, EUS-guided drainage using LAMS (Niti-S HOT SPAXUS; Taewoong Medical Co, Ltd, Ulsan, South Korea) with diameters of 16 mm and 10 mm and with a length of 20 mm is an alternative to be considered since it is technically feasible and effective for treating symptomatic pancreatic pseudocysts, accompanied by few complications when used in our environment.
This study found a shorter hospital stay and an absence of reinterventions. These findings are consistent with the advantages documented from a retrospective cohort study by Akshintala et al.18, in which percutaneous drainage was compared with endoscopic drainage, finding that patients who underwent endoscopic drainage had fewer reinterventions (9.8% versus 42.5%; p = 0.001), shorter length of hospital stay (6.5 ± 6.7 days versus 14.8 ± 14.4 days; p = 0.001) and lower average number of follow-up imaging (4 [2.5-6] vs. 6 [3.25-10]; p = 0.02). Although in the present study, it was impossible to evaluate a possible decrease in the average number of follow-up imaging requirements, hospital stay times, or extra costs due to complications associated with the pathology, the results are encouraging, given the low frequency of complications and reintervention.
In the particular case of the patient without clinical success, the stent mechanism did not fail, but instead, he bled through the splenic artery, which is why there was technical success. However, he presented with bleeding after 24 hours, which required an endoscopic check-up without being able to achieve adequate hemostatic control. Due to suspicion of injury to the splenic vessels, he was taken for surgical management, in which the etiology of the bleeding was found at the level of the gastric wall, so the stent was removed. Subsequent gastrorrhaphy and marsupialization were performed. Bleeding is a complication previously described in the literature. The study by Siddiqui et al.19 found that using LAMS increased the chances of bleeding, probably because the ridges are broad and can lead to erosion of the stent in a vessel when the cavity wall collapses. Particularly in these cases, bleeding can represent a severe complication because the great vessels often cross the cysts, and bleeding will occur in the pseudocyst cavity, followed by retro- or intraperitoneal blood spill. When compared with plastic stents for drainage of pancreatic collections, patients treated with LAMS have higher bleeding rates20, so these procedures should be performed in referral centers by experts in endoscopic intervention and where there is the support of hepatobiliary surgery and interventional radiology21. During the study, early surgical intervention allowed an adequate evolution without postoperative complications and made timely discharge possible. It is also necessary to mention the stent removal time: Ideally, it should be done at four weeks and not at eight weeks, as in the cases described22. However, administrative barriers may occur on the part of health insurers, which prevent timely access and make it challenging to meet these agreed times. Despite the times in the cases described, no associated complications were identified.
The choice of treatment for pancreatic pseudocysts will continue to be a controversial issue from different points of view, among which is the approach, which includes observation, endoscopic drainage guided or not guided by EUS, percutaneous drainage, and surgical interventions. If endoscopically, it includes whether or not a stent is required, the use of plastic or metal stents, and the form of drainage. Regarding the latter, the evidence has shown diverse results on heterogeneous characteristics in the studies. On the one hand, in the research by Bang et al.23, LAMS was compared with plastic stents for walled-off pancreatic necrosis, and no significant differences were observed in the number of procedures performed, treatment success, adverse clinical events, readmissions, stay, and the overall costs of the treatment. On the other hand, in a recent meta-analysis, the technical success, clinical success, and adverse events of LAMS for pancreatic collections (both walled-off pancreatic necrosis and pseudocysts) were 96.2% (95% CI: 94.6-97.4), 86.8% (95% CI: 83.1-89.8), and 20.7% (95% CI: 16.1-26.1), respectively24. Recently, a case report showed endoscopic cystogastrostomy guided or not with endoscopy and LAMS as a viable, safe, effective, and economical therapeutic option in a case of giant pseudocyst of the pancreas25. All of these are findings of interest and are related to the patient’s characteristics, the collection, and the approach, which is why they merit more extensive studies and follow-up, in which the elements to be considered are defined.
There are some limitations of the study. First, this is a retrospective study with a small number of patients, as it is a limited series of 13 consecutive patients who underwent LAMS placement at two institutions. Furthermore, convenience sampling causes a high possibility of bias. It should be mentioned that only descriptive statistics were performed to express our experience with LAMS, and a statistical analysis of the clinical benefit is needed. Another limitation is that it could not be compared with other stents. This study aimed to demonstrate the initial experience in two local referral centers by describing technical feasibility and clinical success. This study is consistent with other LAMS studies16,26-28 regarding its safety and efficacy in managing pancreatic pseudocysts. Due to the satisfactory results, LAMS continues to be used in handling these types of patients. Finally, in the present study, no defined periods were established for follow-up, so outcomes of interest could not be characterized in periods longer than eight weeks.
Specific questions that should be addressed in future research include: What is the appropriate/safe duration between placement and removal of LAMS? What are the ideal radiological and endoscopic follow-up intervals to reduce the risk of migration of the endoprosthesis and its lodging? Moreover, do plastic stents placed through a LAMS affect migration risk, occlusion, or other complications? Systematic application of deliberately developed and refined protocols should help reduce complications of LAMS and allow for safer application of these critical devices as their clinical use expands over time.
Conclusions
In conclusion, the present observational study demonstrates that, in our setting, the use of LAMS is safe and effective in managing symptomatic pancreatic pseudocysts and has a low frequency of complications. The intervention decision must be based purely on the clinical picture by expert personnel and in a center with support from surgery and interventional radiology, among others.











 texto em
texto em