Introduction
Kaposi’s sarcoma (KS) is a multifocal angioproliferative neoplasm associated with human herpesvirus 8 (HHV-8). It is characterized by the formation of localized or disseminated inflammatory lesions predominantly on the skin and mucous membranes, which can also affect internal organs1-3. The epidemiology of the disease varies according to the demographic characteristics and the degree of immunosuppression of the patients, variables that also impact the clinical picture1,2,4. There are four epidemiological forms: classic, endemic, iatrogenic, and epidemic. It was initially reported in the early 1980s in almost a third of patients with acquired immunodeficiency syndrome (AIDS), inferring its relationship with a state of profound immunocompromise. The clinical course is initially indolent but frequently becomes aggressive and involves viscera4. The iatrogenic form is observed in organ transplant recipients and patients with prolonged immunosuppression due to other causes. The incidence of KS in transplant recipients is 400-500 times higher than in the general population. Manifestations are usually localized but may involve organs2,4.
The epidemic variant presents with extensive mucocutaneous and visceral involvement. It begins as macules that later form plaques and later nodules and tumors. The latter involves lymph nodes and visceral organs, mainly respiratory and gastrointestinal. Oral lesions on the palate and gums are common and may result in dysphagia and secondary infections. The gastrointestinal tract is the most common extracutaneous location, and its lesions are usually asymptomatic. Sometimes, they cause abdominal pain, weight loss, nausea, emesis, malabsorption, diarrhea, bleeding, or obstruction, and their presence is confirmed endoscopically2,5-8.
Gastrointestinal involvement (KS-GI) can be present in up to 40% of patients with KS and AIDS6. Furthermore, it is known that 50% of patients with cutaneous and 75% with oral manifestations will develop gastrointestinal lesions in the future9.
The endoscopic appearance of KS is variable, with lesions that can be red maculopapular or polypoid/nodular and darker, and the latter are the most common, mainly in the stomach. In severe disease, it appears as a friable central or ulcerated umbilication8,10,11.
The prognosis of KS-GI is poor, both for the endemic form and those related to immunosuppression, with a 6-month survival of 40%(8,11). Likewise, mortality is higher in patients with CD4 T lymphocyte counts <150 cells/μL12.
Some clinical variables serve as predictors of suffering from KS-GI. Among them, the CD4+ T lymphocyte count <100 cells/μL, men who have sex with men (MSM), and cutaneous KS have a significant relationship with KS-GI, even in asymptomatic patients. Furthermore, endoscopically severe lesions such as the bulky variety and a high number of lesions (≥10) are associated with a CD4+ T count <100 cells/μL and human immunodeficiency virus (HIV) viral load (VL) >10,000 copies/μL, respectively13.
Treatment is dependent on the KS variant. Epidemic KS includes antiretroviral drugs, while iatrogenic forms can be treated with a reduction of immunosuppressive agents. When these measures are ineffective in disseminated or visceral disease, in endemic or classic KS, and without HIV infection, agents directed against the tumor lesion are indicated. The most used are liposomal anthracyclin (doxorubicin, daunorubicin) and paclitaxel2,5,6. Other drugs that are beginning to be relevant due to results in modest clinical studies are imatinib (tyrosine kinase [TK] inhibitor), bevacizumab (antibody against vascular endothelial growth factor [VEGF]), interleukin 12 (IL-12; cytokine that favors the Th1 lymphocyte response), thalidomide, lenalidomide and pomalidomide (immunomodulatory imides), bortezomib (proteasome inhibitor), and β-blocking agents such as propranolol or timolol2.
In the literature, there are series that describe the clinical, endoscopic, and histopathological characteristics of patients with KS-GI; however, these studies were conducted in populations other than Latin Americans7,8,13. In Colombia, some publications have detailed the panorama of epidemic KS, and others report cases of KS-GI14-17. This study characterized the symptoms, immunological status, endoscopic findings, and therapy used in 24 patients with KS-GI who consulted a tertiary care university hospital in southwestern Colombia between 2011 and 2020.
Methods
A cross-sectional descriptive study was carried out based on medical records of patients with KS-GI who attended a high-complexity university hospital in Cali, Colombia, between January 2011 and January 2020 to evaluate the clinical characteristics and endoscopic examinations of affected subjects.
The inclusion criteria were: age >18 years and diagnosis of KS-GI (ICD-10: C46.0, C46.4, B21.0). Those who lacked an endoscopic record were excluded. The institutional ethics committee approved this study before its initiation.
Clinical variables were collected, such as sex, age, origin, pathological history, history of HIV infection or autoimmune diseases, HIV viral load, CD4+ T lymphocyte count, presence of opportunistic infections, and pharmacological history, including highly active antiretroviral therapy (HAART) and chemotherapy. The symptoms reported by the patients in the consultation, the location of the lesion where the histopathological diagnosis of KS-GI was made, and its endoscopic characteristics were also recorded.
All variables collected are described and summarized. The Shapiro-Wilk test was used to determine the distribution of quantitative variables, and these were summarized with measures of central tendency and dispersion according to their distribution. Categorical variables were summarized as proportions.
Results
One hundred thirty-five patients diagnosed with KS were identified, of which 24 met the KS-GI compromise criterion. Twenty-two of them were male (91.7%). The median age was 36 years (interquartile range [IQR] = 29.5-43). These and other fundamental characteristics of the population studied are shown in Table 1.
Table 1 Sample characteristics
| n | % | |
|---|---|---|
| Sex | ||
| Female | 2 | 8.3 |
| Male | 22 | 91.7 |
| Age* | ||
| Median | 36 | |
| Minimum | 29.5 | |
| Maximum | 43 | |
| Comorbidities | ||
| HIV infection | 21 | 87.5 |
| Arterial hypertension | 2 | 8.3 |
| Type 2 diabetes mellitus | 1 | 4.2 |
| Chronic renal failure | 2 | 8.3 |
| Smoking | 1 | 4.2 |
| Inflammatory bowel disease | 1 | 4.2 |
*Age in years. Table prepared by the authors.
Twenty-one patients had HIV infection (87.5%), which constitutes cases of epidemic variant KS. Of them, 19 were receiving HAART (90.4%). Six different antiretroviral therapy regimens were identified; the most common was abacavir + lamivudine + lopinavir + ritonavir.
In the group with HIV infection, two of the 21 patients had undetectable viral loads (9.5%), and six had loads greater than 100,000 copies/mL (28.5%). Regarding the CD4+ T lymphocyte count, 28.6% had a count of less than 50 cells/μL at the time of KS diagnosis, 16.7% had between 50 and 100 cells/μL, and 19% had a count greater than 200 cells/μL.41.6% of the patients suffered from other opportunistic infections; the most frequent were those caused by Cryptococcus spp. (16.7%), Histoplasma capsulatum (12.5%) and Toxoplasma gondii (8.3%). On the contrary, there was no identification of opportunistic microorganisms in 58.3% of the patients (Table 2).
Table 2 Immunological characteristics
| n | % | |
|---|---|---|
| HIV viral load | ||
| Undetectable | 2 | 9.6 |
| 40-100,000 | 5 | 23.8 |
| More than 100,000 | 7 | 33.3 |
| ND | 7 | 33.3 |
| CD4+ T lymphocyte count | ||
| Under 50 | 6 | 28.6 |
| 50-100 | 4 | 19.0 |
| 100-200 | 3 | 14.4 |
| More than 200 | 4 | 19.0 |
| ND | 4 | 19.0 |
| Infections associated with immunosuppression | ||
| Present | 10 | 41.7 |
| Absent | 14 | 58.3 |
| Reported opportunistic infections | ||
| Cryptococcus spp. | 4 | 16.7 |
| Histoplasma capsulatum | 3 | 12.5 |
| Toxoplasma gondii | 2 | 8.3 |
| Pulmonary tuberculosis | 1 | 4.2 |
| Mycobacterium avium complex | 1 | 4.2 |
| Disseminated cytomegalovirus | 1 | 4.2 |
| Molluscum contagiosum | 1 | 4.2 |
ND: no data. Table prepared by the authors.
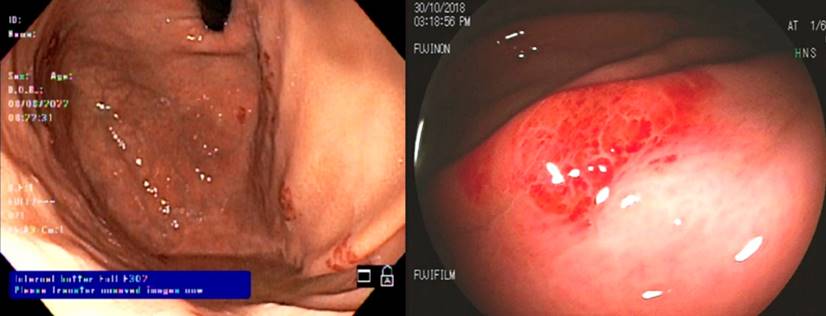
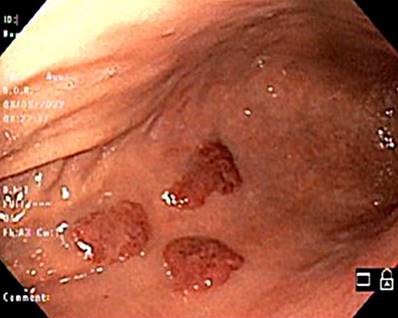
Regarding clinical characteristics, 70.8% of patients with KS-GI had skin lesions, and two-thirds were gastrointestinally asymptomatic. The most frequently reported symptoms were abdominal pain, hematochezia, nausea, and diarrhea for more than a month. The lesions were located most often in the oropharynx (41.7%), followed by the stomach (20.8%), colon (16.7%), small intestine, and rectum (8.3%). The main endoscopic description was maculopapular erythema (Figure 1), followed by nodules (Figure 2) and exophytic lesions. However, the initial report was unavailable in several patients (Table 3).
Table 3 Clinical and endoscopic characteristics
| n | % | |
|---|---|---|
| Skin lesions | ||
| Present | 17 | 70.8 |
| Absent | 7 | 29.2 |
| Gastrointestinal symptoms | ||
| Absent | 16 | 66.7 |
| Present | 8 | 33.3 |
| Symptom | ||
| Abdominal pain | 5 | 20.8 |
| Nausea | 2 | 8.3 |
| Emesis | 1 | 4.2 |
| Diarrhea <1 month | 0 | 0.0 |
| Diarrhea ≥1 month | 2 | 8.3 |
| Constipation <1 month | 1 | 4.2 |
| Constipation ≥1 month | 0 | 0.0 |
| Melenas | 0 | 0.0 |
| Hematochezia | 6 | 25.0 |
| Location of the gastrointestinal involvement | ||
| Oropharynx | 10 | 41.7 |
| Esophagus | 1 | 4.2 |
| Stomach | 5 | 20.8 |
| Small intestine | 2 | 8.3 |
| Colon | 4 | 16.7 |
| Rectum | 2 | 8.3 |
| Endoscopic findings | ||
| Maculopapular erythema | 7 | 29.2 |
| Polyps | 1 | 4.2 |
| Nodules | 3 | 12.5 |
| Exophytic lesions | 3 | 12.5 |
Table prepared by the authors.
In addition to antiretroviral therapy, 58.3% of patients received antineoplastics; doxorubicin treatment was reported in 12 individuals with KS-GI. The presence of other comorbidities in addition to HIV infection was uncommon: one person with type 2 diabetes mellitus, two with chronic kidney disease, two with high blood pressure, and one with inflammatory bowel disease. Additionally, two were smokers, and one patient used corticosteroids chronically.
Discussion
This study sought to characterize the population affected with KS-GI who attended a referral university hospital in southwestern Colombia between 2011 and 2020. The identified patients were mainly early adults, and the predominant epidemiological form of KS was epidemic. Immunological variables such as low CD4+ T cell count and high HIV VL were directly related to KS-GI frequency. The upper gastrointestinal tract was the most involved, and maculopapular erythema was the most frequent endoscopic finding.
The rate of patients diagnosed with HIV infection was 87.5%, very similar to that observed in previous case reports of KS-GI8,13. Within the population evaluated, there was a greater male representation associated with a higher incidence of KS in men than in women and a significant prevalence of HHV-8 infection in MSM2,18-21.
Immunological variables such as low CD4+ T lymphocyte count and high HIV VL are related to KS-GI. A study published by Nagata et al. evaluated 33 patients with KS-GI and found that the majority had CD4+ below 200 cells/μL: 63.7% with CD4 <100 cells/μL and 24.2% between 100 and 199 cells/μL. This same publication reported a positive correlation between patients with KS-GI and high HIV viral loads: 54.6% with VL >100,000 copies/mL and 21.1% with VL between 40 and 10,000 copies/mL13. These data are similar to the present study, in which more than half of the patients had a CD4+ T lymphocyte count <200 cells/μL. In addition, about half of the patients had opportunistic infections. A likely explanation is the high susceptibility to disseminated KS (e.g., gastrointestinal) in scenarios of profound immunosuppression, such as AIDS5,9,12.
Gastrointestinal symptoms were observed in one-third of the subjects evaluated (33.3%); however, other publications report fewer (15-21%)8,13. One hypothesis for this contrast is that non-specific symptoms such as abdominal pain, nausea, and chronic diarrhea predominated in the subjects of our study, which may have causes other than KS-GI in the context of severe immunosuppression and HIV infection. Besides, a high occurrence of KS with cutaneous manifestations was observed among those with gastrointestinal involvement, a finding similar to that of other authors8,13.
The primary location of the lesions was the upper gastrointestinal tract, especially the oropharynx. This information agrees with various cases in which commitment at this level was also more frequent6-9,11,12,22. In the present study, maculopapular erythema was the most repeated endoscopic finding; still, in the literature, the polypoid/nodular lesion is the most common8,13. This difference could be related to the sample size; the endoscopic assessment of lesions is subjective, and there may be variations in the semiological interpretation. Subsequent studies should contrast what is noted in this study.
Only three cases (12.5%) corresponded to non-epidemic KS. In the literature, some studies describe mucosal involvement due to iatrogenic and classic KS with rates of 73.6% and 26.4%, respectively. The most common locations are the oral cavity, stomach, rectum, small intestine, and colon23. In our cohort, there were no details on the topographic characteristics of this group of patients.
This study had several limitations: only 24 subjects with KS-GI were identified over ten years, probably related to underdiagnosis and difficulties accessing highly complex services in Colombia. Furthermore, some patients had previous therapies that could have modified the clinical manifestations during the gastroenterological evaluation. Therefore, an attempt was made to evaluate the records of each patient from the first contact with health personnel (internal medicine, infectious diseases, general medicine, emergency medicine). The proportion of patients with non-epidemic KS-GI was also small. Despite the increased use of various immunomodulators, biological therapies, and organ transplants, epidemic KS is more common than the iatrogenic or classic variant21.
Conclusion
In our study, KS-GI associated with epidemic KS was the most common. The high prevalence of skin manifestations concomitant with lesions in the gastrointestinal tract is highlighted. The findings of the most frequent anatomical sites, the lesions’ main characteristics, and associated factors that can guide the search in this group of patients are described. High HIV viral loads and low CD4 T lymphocyte counts should be considered in the evaluations of patients with immunosuppression since the finding of KS-GI results in a worse prognosis.











 texto en
texto en