Introduction
The ampulla of Vater is located in the second portion of the duodenum at the confluence of the common bile duct and the pancreatic duct of Wirsung. It is surrounded by smooth muscle fibers that make up the sphincter of Oddi1. Different entities can be located at this level and comprise a broad spectrum, from those with benign biological behavior to carcinomas2. The first group includes adenomas, polyps (Peutz-Jeghers syndrome, juvenile polyposis, Cowden syndrome, familial adenomatous polyposis, and Gardner syndrome), lipomas, leiomyomas, vascular abnormalities, and infectious diseases, particularly tuberculosis2. The malignant scenario includes adenocarcinomas, neuroendocrine tumors, lymphomas, stromal tumors, and metastases, to name a few2.
Coccidioidomycosis is a fungal disease endemic to America. It corresponds to a systemic mycosis that predominantly affects people who visit or reside in rural areas with a dry climate, alkaline soil, annual precipitation rates of around 10 cm, and temperatures of up to 50 ºC, favorable environmental conditions for its propagation3,4. Most cases have been reported in the United States and Mexico, and C. posadasii (82%) is the most related etiological agent5.
Patients affected by this disease, in addition to residing in or visiting endemic areas, usually have some degree of immunosuppression due to chronic diseases such as diabetes mellitus. In this scenario, the disease can evolve to severe forms with significant morbidity, characteristics that it shares with tuberculosis, an entity that can manifest synchronously6. Coccidioides infection occurs through inhalation of arthroconidia of the fungus, and in most cases, is usually asymptomatic and can be indistinguishable from influenza infections or other respiratory viruses, with even fatal manifestations7.
In Colombia, there are geographical areas with environmental characteristics conducive to the development of Coccidioides, particularly in La Guajira, Magdalena, and Cesar, located on the northern coast, where an exposure between 3% and 13% of the population has been reported through coccidioidin skin testing5. Only in two of the five Colombian cases described in the literature did the patient have a history of international travel before the development of the disease8,9, and in none of the cases reported to date did the patient have a duodenal mass, jaundice syndrome, and gastrointestinal symptoms.
The present paper seeks to enrich the registry of Colombian cases of Coccidioides. This rare pathology has only been reported five times in the last 60 years10, and to describe an unusual form of clinical manifestation.
Case description
We present the case of a 29-year-old Venezuelan male patient from Santander de Quilichao (Cauca, Colombia) who worked as a deliveryman. For 4 months, he had had pain in the epigastrium, emesis of food and bilious content, soft yellow stools, asthenia, and adynamia. In the last 10 days, he noticed a yellow tinge not associated with acholia or choluria, and in the previous 2 days, a single unquantified fever spike, in addition to the weight loss of 16over the previouslast 4 months. During interrogation, he denied any medical history. In the physical examination on admission, the vital signs were within normal ranges, the sclerae were icteric, the abdomen was painful on palpation of the epigastrium without signs of peritoneal irritation, and there was non-painful mobile bilateral inguinal lymphadenopathy. Blood tests showed serum polymerase chain reaction (PCR): 238 mg/dL, total bilirubin: 8.5 mg/dL, direct bilirubin: 4.9 mg/dL, alkaline phosphatase: 2013 UI/L, amylase: 64 U/L, alanine aminotransferase (ALT): 324 IU/L, aspartate aminotransferase (AST): 168 IU/L and blood count with leukocytes: 10,700/μL, hemoglobin: 12.3 g/dL, hematocrit: 37.7%, platelets: 467,000/μL and neutrophils: 79.8%. The total abdominal ultrasound showed dilation of the intra- and extrahepatic bile duct with an 11 mm common bile duct, without lesions in the liver parenchyma and multiple peripancreatic lymphadenopathy. Subsequently, a simple and contrast-enhanced abdomen CT scan was ordered, confirming a mass at the periampullary level associvarioush multiple mesenteric and retroperitoneal lymphadenopathy (Figure 1).
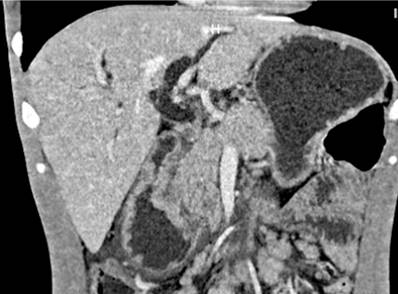
Figure 1 Simple and contrast-enhanced abdominal CT scan: dilation of the intra- and extrahepatic bile duct with a 14-millimeter common bile duct, the pancreas with slight dilation of the Wirsung duct, and loss of contours at the level of the head. The concentric circumferential wall thickened with enhancement after contrast administration and multiple mesenteric, retroperitoneal, latero-aortic, and intercaval-aortic lymphadenopathy in the periampullary region. Source: Radiology Department, Hospital Universitario del Valle.
The CA 19-9 and carcinoembryonic antigen were negative (less than 1.4 U/mL and 1.21 μg/L, respectively), as was the PCR test for severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2). Given the imaging characteristics, paraclinical findings, and clinical history, an endoscopic study was considered, which revealed esophageal moniliasis (KOSI III classification) and a tumor-like lesion in the second portion of the duodenum with stricture of 80% of the lumen. A test for HIV was performed, which was positive. The patient continued under medical management with intravenous fluids and analgesia with clinical and paraclinical improvement, so the study continued on an outpatient basis with antiretroviral therapy, fluconazole, and ivermectin, and he was summoned for a check-up with the results. However, the patient has not attended clinical follow-up.
The anatomopathological study of the endoscopic biopsy of the lesion identified acute and chronic ulcerated duodenitis with a granulomatous component and abundant blastoconidia compatible with Coccidioides spp (Figure 2).
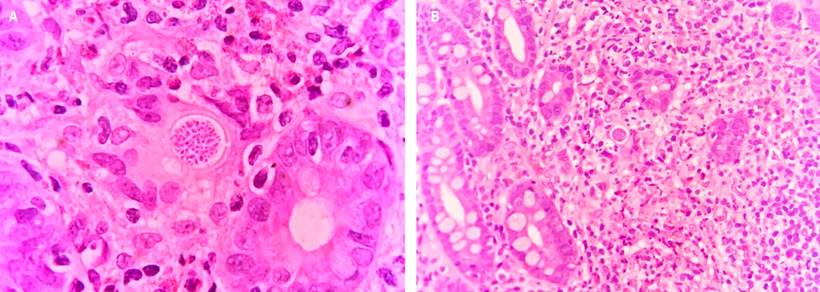
Figure 2 Spherules with endospores in a duodenal mucosa biopsy with mixed inflammatory infiltrate with neutrophils, epithelioid histiocytes, and eosinophils. Source: Pathology Department, Hospital Universitario del Valle.
During the clinical stay, it was considered that he most likely had a neoplasm of epithelial or hematolymphoid origin, and he was discharged for outpatient follow-up with an order for magnetic resonance cholangiography. Still, there were no follow-up data at the time of publication.
Discussion and literature review
Disseminated coccidioidomycosis is a rare disease, and although Colombia has some areas with ideal environmental conditions for the proliferation of the microorganism, only five cases have been reported in the last 70 years10, two of which are related to the migrant population and the rest with the indigenous people. The case presented in this article is the sixth reported to date10; however, under-registration and difficulties in diagnosing it11 (biosafety standards for culture and identification of Coccidioides or serological diagnosis) may mean that the local casuistry is poorly nourished.
Patients with this fungus infection are suspected to be immunosuppressed12 (HIV, organ transplant, use of immunomodulatory medications) and reside or have traveled to endemic areas, as is the case of the patient in question. Clinical manifestations fluctuate in a broad spectrum, from asymptomatic subjects (60%) to those requiring management in the ICU and even death13. When referring to disseminated mycosis, the manifestation is just as variable as the clinical course; skin, lymph nodes, bone, and central nervous system involvement may be found. Still, practically any organ or system can be affected13. Patients’ different clinical and sociodemographic conditions have been related to less favorable scenarios. People with diabetes, tobacco users, and adults over 65 years of age exposed to biomass or archaeological sites have a higher risk of severe lung infection12. Ethnic origin seems to be a risk factor for severe disease and systemic dissemination: The Caucasian and African-American populations have significantly higher rates of these clinical manifestations13.
The extrapulmonary manifestation has been reported in less than 1% of cases in immunocompetent patients and up to 50% of immunocompromised patients14, as in the case presented, who had de novo HIV. Gastrointestinal involvement is rare and is more likely related to hematogenous transition or ingestion of secretions15.
Coccidioidomycosis is diagnosed by identifying the fungus in the anatomopathological study of the affected tissue, cultures, or PCR assays. The latter can be performed on fresh tissue and tissues fixed in formalin16,17. Furthermore, some tests are currently used preferentially in the epidemiological and research field since serological tests have high rates of false positives (up to 20%) and doctors are not familiar with their correct interpretation18,19.
Treatment of disseminated disease requires fluconazole at doses of 800 to 1,200 mg/day20,21. This medication has response rates of approximately 79%21. Nonetheless, lifelong prophylactic use is recommended in immunosuppressed patients due to the high rate of relapses and associated mortality22.
Conclusions
Although coccidioidomycosis is an endemic disease in America, Colombia has a prevalence that is well below that of Venezuela and Brazil, probably due to under-reporting and difficulties in diagnosis, in addition to the country’s environmental characteristics, which limit the macroenvironment favorable for the proliferation of Coccidioides to the northern region of the country.
This case is of particular interest because it initially manifested as a periampullary mass with high suspicion of neoplasia and rapidly evolving jaundice syndrome, an unusual and unique manifestation according to previous cases reported in Colombia, which corresponded to pulmonary forms.
Mycosis due to this agent in Colombia has a low prevalence. The proximity to Venezuela and the high flow of migrants of this nationality should expand the spectrum of differential diagnoses in the evaluation of patients with this symptomatology.











 texto em
texto em 



