Clinical case
We present the case of a 35-year-old man of African descent, born on the Colombian Pacific coast, with no relevant history. He consulted due to a one-year-old clinical picture of diffuse abdominal pain, which was later located in the right hypochondrium. He also had persistent objective fever for three months, unquantified weight loss, and predominantly right-sided, subcentimeter, non-painful axillary lymphadenopathy. On physical examination, no masses or organomegaly were found. He denied recent travel or use of allopathic or homeopathic medications.
Within the initial diagnostic approach, an infiltrative hepatic biochemical pattern drew attention (Table 1). Hepatobiliary ultrasound ruled out the intra- or extrahepatic bile duct dilation without masses or collections in the liver parenchyma. Given the persistence of fever, a contrast-enhanced computed axial tomography (CT) of the abdomen was requested, noting multiple hypodense focal lesions in the right hepatic lobe, not suggestive of abscesses (Figure 1).
Table 1 Basic biochemistry
| Parameter | Result | Reference value |
|---|---|---|
| Leukocytes | 7620 | 4500-10 000/µL |
| Neutrophils | 4750 | 1400-6500/µL |
| Lymphocytes | 2240 | 1200-3400/µL |
| Eosinophils | 70 | 0-700/µL |
| Monocytes | 340 | 0-1200/µL |
| Basophils | 50 | 0-200/µL |
| Hemoglobin | 15 | 12.5-16 g/dL |
| Platelets | 246 000 | 150 000-450 000/µL |
| PCR | 42.9 | 0-5 mg/L |
| Procalcitonin | 0.087 | 0 ng/mL |
| Direct bilirubin | 1.81 | 0-0.3 mg/dL |
| Indirect bilirubin | 0.04 | 0-1.1 mg/dL |
| Total bilirubin | 1.85 | 0.2-1.3 mg/dL |
| ALT | 41.2 | 14-54 U/L |
| AST | 40.8 | 15-41 U/L |
| Alkaline phosphatase | 701 | 38-128 U/L |
| PT | 11.3 | 10.1-11.8 s |
| Albumin | 4.4 | 3.5-5.2 g/dL |
| ECA | 31.7 | 9-97 U/L |
| Calcium in urine 24 hours | 197.87 | 100-249 mg/24 h |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; ACE: angiotensin-converting enzyme; CRP: C-reactive protein; PT: prothrombin time. Prepared by the authors.
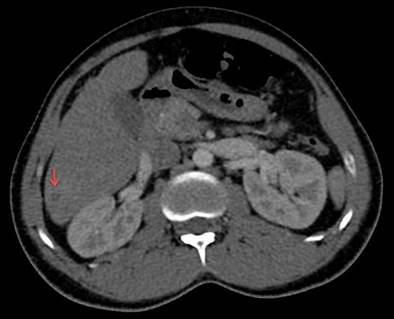
Figure 1 Contrast-enhanced abdominal CT (axial section). Hypodense lesions are observed in the right hepatic lobe, with peripheral enhancement with a contrast medium of up to 26 x 29 mm. Gallbladder and intra- and extrahepatic ducts with typical characteristics (red arrow). Courtesy of the Servicio de Radiología e Imágenes Diagnósticas, IDIME. Consorcio Clínica Nueva Rafael Uribe Uribe. Cali, Colombia.
After two weeks of inconclusive studies, it was decided to take him for a liver biopsy (Figure 2). PAS, Gomory, Grocott for fungi, Ziehl-Neelsen for acid-fast bacteria (AFB), and Gram and Warthin-Starry stains were negative, as were multiple polymerase chain reaction and culture in liquid medium for Mycobacterium tuberculosis, with an incubation time of 42 days.
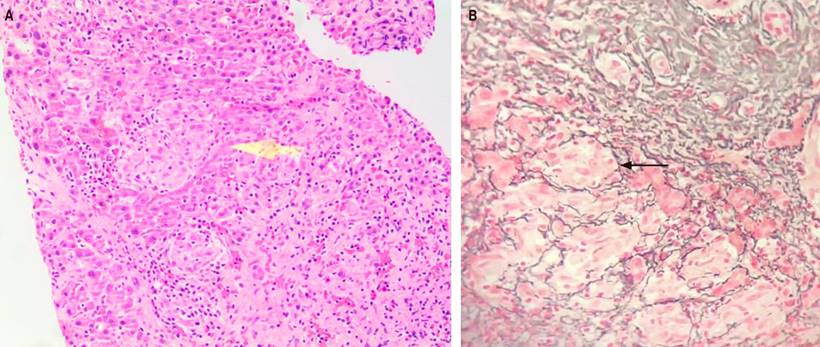
Figure 2 Liver biopsy. A. The sections show the liver parenchyma with numerous small granulomas, which are frequent in the periportal region, consisting of multiple compact aggregates of epithelioid cells with little lymphocytic infiltrate around them, without necrosis (hematoxylin and eosin, 100x); no fungi or mycobacteria were observed in the PAS and Ziehl-Neelsen stains (yellow arrow). B. In the reticulum stain, a cuff of fibrosis is noted around the granulomas (black arrow). The morphological findings correspond to granulomatous hepatitis (silver salt stain, 40x). Courtesy of Dr. María Mercedes Mendoza, pathologist, Hospital Militar Central. Bogotá, Colombia.
Taking into account the ethnic group, the periportal distribution of the granulomas, and the absence of caseation in them, sarcoidosis was considered a differential diagnosis, for which 24-hour urine calcium and angiotensin-converting enzyme (ACE) levels were requested (despite its low sensitivity). It was complemented with a high-resolution CT (HRCT) of the chest (Figure 3) and a bronchoalveolar lavage by fiberoptic bronchoscopy with negative cytological and microbiological studies.
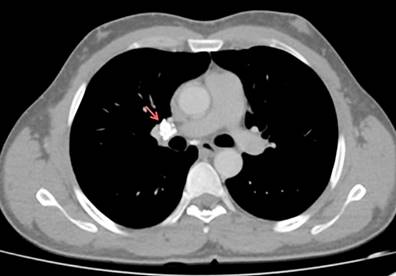
Figure 3 Contrast-enhanced chest CT (mediastinal window). Calcified subcarinal lymphadenopathy measuring 12.9 mm x 12.8 mm and residual right hilar lymphadenopathy are shown (red arrow). Courtesy of the Servicio de Radiología e Imágenes Diagnósticas, IDIME. Consorcio Clínica Nueva Rafael Uribe Uribe. Cali, Colombia.
Studies were expanded for infectious, autoimmune, and miscellaneous causes in the context of fever of unknown origin (Tables 2 and 3). Although the first report of the Mantoux test (PPD) was 0 mm, a second study carried out two weeks later was interpreted as 9 mm (a booster effect in a probable case). Thus, it was decided to start tetraconjugate treatment with HRZE (H: isoniazid, R: rifampicin, Z: pyrazinamide, E: ethambutol) for at least two months given the availability of interferon-gamma release assay (IGRA) in the context of probable tuberculosis with localized liver involvement despite the absence of confirmatory microbiological studies (negative cultures for M. tuberculosis in bronchoalveolar lavage and liver tissue sample) and calcified lymphadenopathy in the mediastinal window of HRCT of the chest.
Table 2 Microbiological studies
| Parameter | Result | Reference value |
|---|---|---|
| Tuberculin (PPD); second sample | 9 mm | < 10 mm |
| Blood cultures #3 | Negative | |
| Detection of M. tuberculosis (PCR) in paraffin block | Not detectable | |
| Gram stain in BAL | Negative | |
| Baciloscopy in BAL | Negative | |
| KOH to BAL | Negative | |
| Cultivation for fungi in BAL | Negative | |
| Culture for mycobacteria in BAL and liver tissue sample | Negative after 42 days | |
| Toxoplasma gondii, IgM antibody | Non-reactive | |
| Toxoplasma gondii, IgG antibody | Non-reactive | |
| Toxoplasma IgG avidity test | Non-reactive | |
| Febrile antigens | Negative | |
| Rickettsia rickettsii IgG | Non-reactive | |
| Rickettsia rickettsii IgM | Non-reactive | |
| Coxiella burnetii phases 1 and 2, IgG antibodies | Non-reactive | |
| HBsAg | Non-reactive | |
| Anti-HCV | Non-reactive | |
| ELISA for HIV ½ | Non-reactive | |
| Anti-Brucella IgM antibodies | Negative | |
| Anti-Brucella IgG antibodies | Negative | |
| Total anti-Brucella antibodies, Rose Bengal Test | Under 25 |
Anti-HCV: antibodies against hepatitis C virus; ELISA: enzyme-linked immunosorbent assay; FBC/BAL: fiberoptic bronchoscopy + bronchoalveolar lavage; HBsAg: hepatitis B surface antigen; IgG: immunoglobulin G; IgM: immunoglobulin M; KOH: potassium hydroxide; PPD: purified protein derivative skin test; HIV: human immunodeficiency virus. Prepared by the authors.
Table 3 Autoimmunity studies
| Parameter | Result | Reference value |
|---|---|---|
| ANA | 1:160 AC-4 pattern | < 1:80 |
| Antineutrophil cytoplasmic antibodies (ANCA-c and ANCA-p) | Non-reactive | |
| AMA | Non-reactive | |
| Complement C3 | 252 | 88-165 mg/dL |
| Complement C4 | 58.2 | 14-44 mg/dL |
AMA: antimitochondrial antibodies; ANA: antinuclear antibodies. Prepared by the authors.
The patient showed improvement in symptoms (disappearance of feverish spikes and abdominal pain) and normalization of the liver biochemical profile 14 days after the start of empiric anti-tuberculosis therapy without any systemic steroids, as initially planned. To date, he is being followed up on an outpatient basis by internal medicine and infectious diseases, with an excellent clinical response and normalization of the liver biochemical profile.
Discussion
The approach to a fever of unknown origin will always be challenging for the clinician. Although up to 70% of cases are due to infectious processes, sometimes the clues are confusing, which requires studies according to dynamic details of both the physical examination and the diagnostic aids, among which the liver biopsy stands out1. It is beneficial in countries considered endemic for tuberculosis, such as Colombia, where the prevalence reaches 26 cases per million inhabitants, with an incidence rate of 22.6 cases per 100,0002. Its extrapulmonary manifestation occurs in up to 15% of patients and is associated with pulmonary involvement at the time of manifestation in about 11%. The prevalence of liver involvement in active tuberculosis is estimated at 1%2,3.
Around 15% of liver biopsies will report granulomatous hepatitis, an entity that covers a wide range of infectious or non-infectious entities. This last category includes autoimmune, drug-induced toxicity, or idiopathic causes. Epidemiology depends on the geographical area and the sociodemographic conditions of the population. Coash et al.4 reported that 66% of cases were secondary to a systemic disease, 28% to primary liver disorders, and 6% to idiopathic. Sarcoidosis, mycobacterial infection, primary biliary cholangitis, and drug-induced hepatotoxicity account for 75% of the total causes described3,5.
In epithelioid granulomas (homogeneous differentiation of activated macrophages into cytokine-secreting cells, without inclusions), the presence or absence of necrosis will guide a directed clinical approach. After a systematic study that allows the most frequent infectious and non-infectious causes to be excluded, between 13% and 36% of cases will be considered idiopathic, which is associated with a good prognosis5.
Depending on some specific cell groups, it is possible to narrow the diagnostic threshold. Thus, the presence of plasma cells in the lymphocytic mantle suggests liver involvement due to syphilis. At the same time, numerous eosinophils could indicate hypersensitivity to drugs or a parasitic disease such as schistosomiasis6. In the case of tuberculosis with liver involvement, the formation of more complex granulomas (immune granulomas) composed of macrophages transformed into epithelioid histiocytes surrounded by T and B lymphocytes differentiated into plasma cells is described in most cases6,7; however, based on extensive case series, it is preferred to classify hepatic involvement due to tuberculosis in two scenarios: as part of a miliary manifestation and as localized disease, which can, in turn, be divided into focal or nodular tuberculosis (including liver abscess or tuberculomas) and in the tubular form (involvement of the intrahepatic duct). The clinical spectrum of localized hepatic tuberculosis ranges from tuberculous liver abscess to tuberculous pseudotumor, primary hepatic tuberculosis, tuberculous hepatitis, tuberculous cholangitis, and bile duct tuberculosis, causing some confusion in the classification and clinical meaning of this disease. Accordingly, a practical classification of hepatic tuberculosis proposed by Álvarez in 19988 divides it as follows:
Miliary tuberculosis (50-80% of cases) consists of liver involvement as part of generalized miliary tuberculosis, without signs or symptoms relevant to the liver.
Tuberculous hepatitis presents with unexplained fever, with or without mild jaundice and hepatomegaly, caseating or non-caseating granulomas in liver biopsy, and improvement with antituberculous treatment.
Hepatobiliary tuberculosis presents with signs and symptoms relevant to the hepatobiliary tract. It includes two subtypes: the first without involvement of the bile ducts, which manifests as solitary or multiple nodules, tuberculomas, and tuberculous liver abscesses, and the second with involvement of the bile ducts that causes obstructive jaundice, either due to the increase in size of the nodes that surround the bile ducts or due to granulomatous involvement of the duct wall that produces inflammatory stenosis. It also includes primary hepatic tuberculosis in the form of a mass, which simulates an intrahepatic carcinoma or a hilar cholangiocarcinoma; hence, the importance of histopathological diagnosis.
The location of granulomas in the pathology sample can guide the diagnosis. For example, in cases of primary biliary cholangitis and sarcoidosis, they are observed near the portal triads. At the same time, drug-induced granulomas are often poorly defined and are found within the hepatic lobes4,9. In the case of liver involvement due to tuberculosis, the location of granulomas is variable. It can extend throughout the lobe, frequently near the portal triads, where they tend to unite even in the centrilobular areas. Furthermore, the morphology of granulomas is nonspecific, considering that other granulomatous diseases can produce similar lesions in the wall of the central hepatic vein7,9.
Finally, in the scenario of epithelioid granulomas, the presence or absence of necrosis, as well as caseum, will decisively guide the diagnostic and therapeutic approach over time. In the case of tuberculosis, as granulomas increase in size, central caseous necrosis may develop with the formation of a capsule around them after transforming histiocytes into fibroblasts. Acid-fast stains or cultures are generally positive in 0% to 59% of liver biopsies10. Still, mycobacteria are more likely to be found in granulomas with caseous necrosis6,9, which explains the negative microbiological studies in our case, considering the histopathological findings described10.
Cultures provide the most significant evidence of liver tuberculosis, but the sensitivity may be less than 10%9,10. Likewise, the PCR for the DNA of M. tuberculosis has a sensitivity of 53% to 88% and a specificity of 96% to 100% to detect liver tuberculosis, so a negative report does not rule out this diagnosis10. Some patients with tuberculosis may have negative PCR results of liver tissue due to the paucity of mycobacteria or the possible reactive nature of liver granulomas8,10.
For its part, sarcoidosis is a chronic granulomatous disease characterized by non-caseating epithelioid granulomas that affect multiple body organs. It frequently affects the lungs, lymph nodes, and liver but can affect any organ in the body. Liver involvement due to sarcoidosis is at least twice as common in African Americans than in Caucasians and occurs in between 11% and 80%, the majority asymptomatic11.
Between 50% and 79% of liver biopsies in patients with sarcoidosis show evidence of hepatic granulomas. The most common symptoms are abdominal pain and pruritus. Only 10% present with hepatomegaly, and less than 5% present with isolated jaundice10; portal hypertension is observed in up to 3% of cases11,12. Hepatic sarcoidosis has three histological categories: cholestatic, necroinflammatory, and vascular12. Histology typically reports non-necrotizing epithelioid granulomas, although necrotizing granulomas have been described as sarcoidosis12,13.
In recent decades, significant advances have been made to define sarcoidosis’s clinical, radiological, immunological, and pathological characteristics, considering it is a diagnosis of exclusion. The diagnosis of sarcoidosis is based on three main criteria: clinical manifestation compatible with sarcoidosis, presence of non-necrotizing granulomatous inflammation in one or more tissue samples, and exclusion of alternative causes of granulomatous disease11,14, and its diagnosis in developing countries with a high burden of tuberculosis, where it is often the last option, is a true challenge.
Nonetheless, when caseous necrosis is not noted, and acid-fast staining of biopsy samples is negative, a patient with suspected tuberculous infection may be misclassified as sarcoidosis15, so it is suggested to rely on histopathological studies, given that the predominant cavitary lesions in the upper lung lobe favor tuberculosis diagnosis, a finding occurs in only 3% of sarcoidosis cases15,16. Similarly, it should be remembered that the reported cases of hepatic sarcoidosis initially describe lung involvement due to sarcoidosis17, unlike tuberculosis, which can have an organ-specific manifestation, even outside the hematogenous dissemination typical of the miliary form.
In our case, we used clinical judgment to opt for a case of tuberculosis with isolated liver involvement after ruling out sarcoidosis following current guidelines17,18, considering that it was a probable scenario of latent M. tuberculosis infection in a country with an intermediate-high incidence for it, which also explains the finding of non-caseating granulomas and the impossibility of definitive microbiological isolation. The histopathological findings that allow us to discern between sarcoidosis19 and other granulomatous diseases (especially tuberculosis and infections due to nontuberculous mycobacteria) are listed below, with the clarification that there are no pathognomonic descriptions for each entity (Table 4).
Table 4 Anatomopathological keys for the diagnosis of sarcoidosis19,20
| In favor of sarcoidosis | Against sarcoidosis |
|---|---|
| Presence of granulomas | |
| - Numerous | - Few granulomas |
| - Absent, but with hyalinized nodular fibrosis suggestive of a resolved granuloma (scattered multinucleated giant cells can be detected) | - Missing |
| Granuloma morphology | |
| - Compact, tightly formed collections of large “epithelioid” histiocytes and multinucleated giant cells. Granulomas tend to be non-necrotic or focal or with minimal ischemic necrosis | - Loosely organized collections of mononuclear phagocytes/multinucleated giant cells |
| - Fibrosis starting in the periphery with a central extension of the granuloma with or without calcification | - Extensive necrosis |
| - Dirty necrosis (with nuclear waste) | |
| - Spliced granulomas | |
| Location of the lesion | |
| - Perilymphatic: around broncho-vascular bundles and fibrous septa containing pulmonary veins and nearby visceral pleura | - Lack of lymphangitic distribution |
| - In sarcoid angiitis and necrotizing granulomatosis: granulomatous angiitis with invasion of the vascular wall | - Intraalveolar granulomas |
| - Scant surrounding lymphocytic infiltrate | - Robust surrounding inflammatory infiltrate (includes lymphocytes, neutrophils, eosinophils, and plasma cells) |
| - Secondary lymphoid follicles | |
| Stains and culture | |
| - Negative | - Positive |
| Multidisciplinary clinical features | |
| - Intra- and extrathoracic involvement | - Extrathoracic involvement only |
Taken from: Crouser ED et al. Am J Respir Crit Care Med. 2020;201(8):e26-51; Lim EJ et al. Med J Aust. 2008;188(3):166-7.
Lastly, a diagnostic algorithm is proposed for the systematic approach to granulomatous hepatitis, which includes everything from the incidental finding in the patient with persistently elevated alkaline phosphatase to the overlooked spectrum of fever of unknown origin in tropical countries, such as ours (Figure 4).
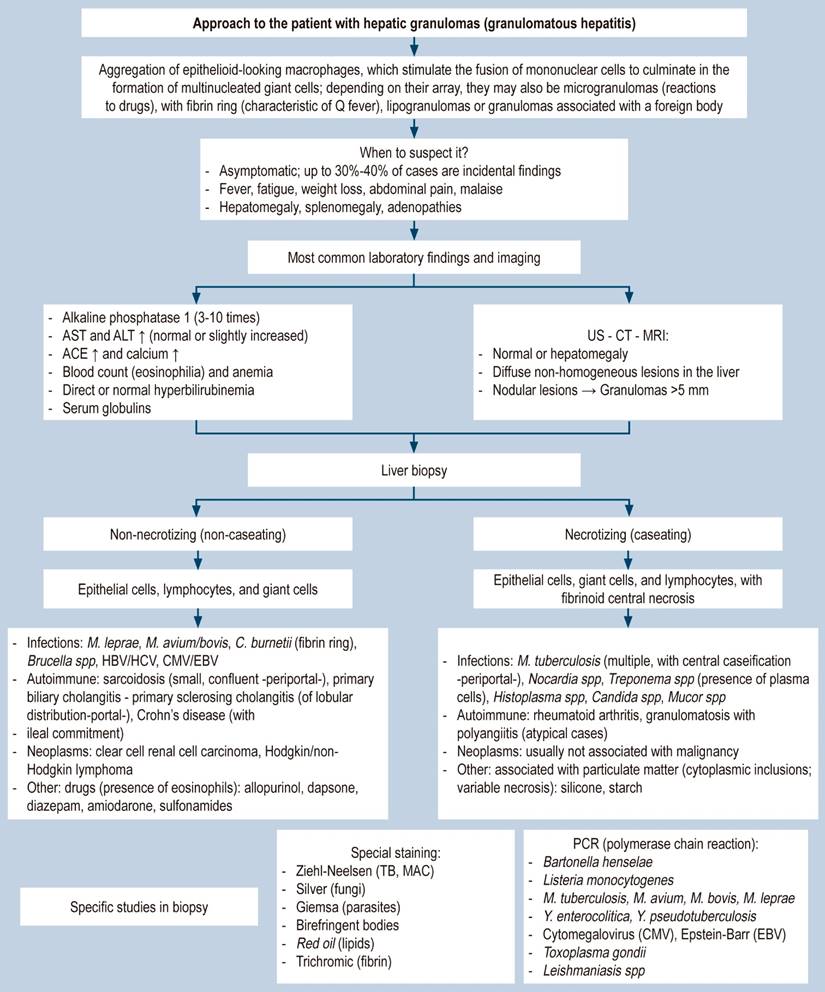
Figure 4 Approach to the patient with granulomatous hepatitis. AST: aspartate aminotransferase; ALT: alanine aminotransferase; ACE: angiotensin-converting enzyme; MAC: Mycobacterium avium complex; MRI: magnetic resonance imaging; CT: computed axial tomography; TB: tuberculosis; HBV: hepatitis B virus; HCV: hepatitis C virus. Prepared by the authors.
Conclusions
Granulomatous hepatitis is an entity that integrates infectious, autoimmune, neoplastic, and toxic causes. It is essential to rule out M. tuberculosis infection in developing countries since empirical immunosuppressive therapy in case of active infection can be catastrophic. Localized liver involvement can occur in primary infection, in which case there is no evidence of previous infection or as reactivation of latent tuberculosis in the form of non-caseating, paucibacillary granulomas, which may reside in the lobules or portal tracts.
Liver involvement in tuberculosis usually implies hematogenous dissemination; however, there are case reports of organ-specific involvement in the liver. In suspected cases, it is suggested to perform combined studies in liver biopsy: Ziehl-Neelsen for AFB, cultures, and PCR amplification for M. tuberculosis to increase sensitivity and specificity (80% and 100%, respectively), although the patient’s country of origin and the time spent there before emigrating determines the risk of tuberculosis. If the diagnostic suspicion persists despite negative direct microbiological tests (direct, culture, and PCR). In that case, we suggest complementing with tests mediated by cellular immunity, considering that both the Mantoux test and the IGRA can be negative for up to 20% of active tuberculosis cases. This applies especially when there is fever of unknown origin, which is often due to latent forms of tuberculosis in subjects with or without underlying immunosuppressive conditions.
In developing countries, we recommend culturing M. tuberculosis samples compatible with granulomatous hepatitis. Once fixed in formalin and paraffin, the tissue cannot be cultured. Still, PCR for M. tuberculosis can yield a delayed diagnosis: AFB or granulomas with caseous necrosis are not always observed. If tuberculosis cannot be excluded, a trial of conventional anti-tuberculosis tetraconjugate therapy for two months to one year can be justified, with strict monitoring of the patient’s hepatic biochemical profile and clinical condition.











 text in
text in 



