Introduction
Anal intraepithelial neoplasia (AIN), or squamous intraepithelial lesion (SIL), is a premalignant lesion. It is a precursor to squamous cell carcinoma, strongly associated with oncogenic strains of the human papillomavirus (HPV), specifically subtypes 16 and 18, risk factors due to behaviors associated with the acquisition of this virus, such as human immunodeficiency virus (HIV) infection, smoking, receptive anal intercourse, and a history of cervical intraepithelial neoplasia (CIN)1.
Anal squamous cell carcinoma (ASCC) is rare, and among specific high-risk populations, its incidence may exceed that of colon cancer. Patients with inflammatory bowel disease (IBD) have a higher risk than the general population: An incidence of 5.5 per 100,000 patients has been identified in the group with IBD compared to 1.8 in the group without IBD2.
It is known that the prevalence of AIN, as well as its progression to carcinoma of the anal canal, is higher in immunosuppressed patients and associated with a prolonged inflammatory state. It favors the persistence and decreases the viral clearance of HPV in the transition zone of the anal canal, increasing the possibility of developing premalignant lesions and, consequently, anal cancer. Besides, prolonged management with anti-tumor necrosis factor (anti-TNF) and steroids causes a loss of cell cycle regulation, which alters viral clearance.
It is possible to detect precursor lesions of anal cancer mainly through anal cytology and high-resolution anoscopy (HRA)3. Currently, there are no recommendations for HPV screening of the anal canal in patients with IBD. Therefore, we conducted a study to confirm the presence of premalignant lesions related to HPV in this area that could explain the high risk of cancer in these patients and develop a screening algorithm in which HRA is the cornerstone for the diagnosis and treatment of premalignant lesions4.
At present, surveillance protocols define that cytology or HRA is restricted to men who have sex with men (MSM), HIV carriers, recipients of solid organ transplants, and patients using immunosuppressive medications such as chemotherapeutics and steroids, among others5,6.
This work aims to reveal the incidence of dysplasia and ASCC in a series of cases of patients who suffer from IBD and are at potential risk of developing these conditions7-9.
Methods
A descriptive case series study was conducted with patients under follow-up for IBD, without perianal symptoms, between January 2022 and July 2022 at the Instituto de Coloproctología ICO S. A. S. in Medellín. They underwent anal cytology, HPV genotyping, and HRA after explanation and acceptance of the procedure. If lesions were found, ablative treatment was performed.
Results
We included a total of 31 patients with ulcerative colitis (UC; 10 women [47%] and average age of 35.2 years [19-58 years]) with an average disease duration of 4.4 years (0.5-12 years) and an extension of colitis according to the Montreal classification: E1: 7 (17%), E2: 8 (18.5%), and E3: 6 (14.5%).
Thirty-two percent (10) of the patients were under follow-up with conventional therapy (mesalazine, azathioprine). Notably, 6 (19%) of the patients required steroids (prednisolone) in the last year, with an average duration of 6 weeks. The remaining 68% (21) presented with failure of the first management line and were under treatment with biologics (20 with anti-TNF and 1 with vedolizumab).
Cytology was positive for HPV in 23 patients (74.2%). We found atypical squamous cells of undetermined significance (ASCUS) in 10 (32.3%) patients, low-grade squamous intraepithelial lesion (LSIL) in 8 (25.8%), high-grade squamous intraepithelial lesion (HSIL) in 5 (16.1), and normal results in 5 (16%). Cytology was not performed in 3 (1%). HPV genotyping was positive in 28 (90.3%) patients, of which 76.1% were high-risk strains. During anoscopy, dysplasia was found in 11 patients (35.5%), condylomas in 5 (16.1%), and normal results in 15 (48.4%).
When the anoscopic findings were positive for dysplasia/condylomas, they contrasted with concomitant medication. Dysplasia and anti-TNF use was found in 9 (56%) patients, dysplasia and a history of steroid use in 8 (50%), condylomas and anti-TNF use in 5 (31.3%), and condylomas and a history of steroid use in 7 (44%). Importantly, there were no positive anoscopic findings in patients under conventional therapy or vedolizumab.
So we must ask: Should we add a new risk population group to the literature?
Discussion
What is the reality of anal cancer?
In Colombia, no national record of neoplasms allows us to describe the incidence of ASCC. Still, it is a rare neoplasm that constitutes less than 5% of all gastrointestinal cancers.
According to recent publications, it has been seen that in healthy men, it went from 0.8 cases per 100,000 inhabitants in the early 1990s to 1.3 cases per 100,000 by 2012. There is also a more proportional growth in women who have suffered cervical neoplasms. According to data for 2017 from the Surveillance, Epidemiology, and End Results (SEER), a program of the National Cancer Institute (NCI) in the United States, there were about 10,000 cases yearly.
A global incidence is estimated at 1.8 cases per 100,000 individuals, increasing along with mortality; 90% of cases are related to HPV infection. In fact, few doctors know that anal cancer is more common in women in the general population than in men. If the groups commonly called high-risk are analyzed, such as HIV-infected patients or MSM, the figures for anal cancer are exponentially higher than in the general population (1.8/100,000). The incidence of anal cancer in MSM with HIV (-) is 35/100,000, while in MSM with HIV (+) it is 131/100,00010.
What is HPV, and why is it an oncogene?
HPV is a small, non-enveloped papovavirus with double-stranded DNA, whose sexually transmitted infection is the most common pathogen in humans11. It is now recognized that HPV infection is responsible for almost all cervical cancers, 95% of anal cancers, 65% of vaginal cancers, 50% of vulvar cancers, and 35% of penile cancers, as well as a significant number of head and neck cancers. Almost 63% of new cases and 61% of deaths will occur in women1.
There are about 200 subtypes based on the genetic sequence of the major capsid protein L1. Approximately 40 subtypes are transmitted by contact between mucosal epithelia, and subtypes 16, 18, 31, 33, 35, 52, and 45 are high risk; they represent the most critical carcinogenic group involved in ASCC: they are isolated in 91% of these tumors, but only serotypes 16 and 18 are responsible for 79% of anal cancers12.
HPV infects keratinocytes and incorporates the E6 and E7 oncogenes into the host genome. These oncogenes subsequently induce the degradation of p53 and Rb, two crucial tumor suppressor proteins typically found in cells, and the inactivation of retinoblastoma family products, ultimately resulting in chromosomal or microsatellite instability. If these processes are not controlled without these proteins, normal cells can mutate into cancer cells following a sequence from dysplasia to neoplasia10.
The Centers for Disease Control (CDC) suggests that “more than 80% of sexually active people will be infected with at least one HPV serotype at some point in their lives.” It is estimated that, worldwide, one million people become infected daily, with a prevalence of 79 million.
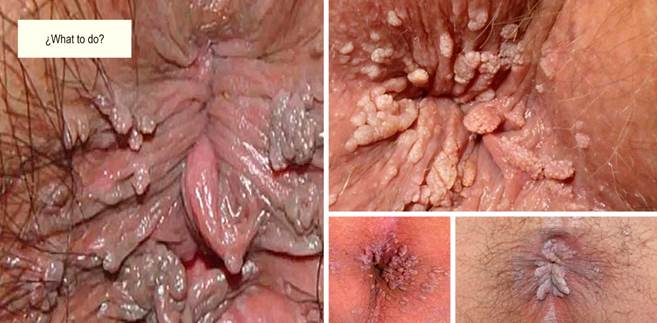
In the vast majority, HPV is asymptomatic, and the infection often disappears on its own thanks to cellular scavenging and shedding mechanisms. However, it can also be subclinical, microscopically detectable (anal cytology/HRA), or latent (i.e., an infection that manifests clinically months or years after exposure), but also macroscopically evident (condyloma) (Figure 1). HPV infection can also start in one part of the body and then migrate to another part (initially beginning in the genitals and then infecting the anus, without necessarily having occurred at this level).
A study of 431 women found that 42% were positive for anal HPV DNA at enrollment, but after a 1.3-year follow-up, that number rose to a total of 70%, and 50% of these developed anal HPV infections during this period, but 58% also cleared their infections during the subsequent follow-up period13.
Moreover, the risk of anal cancer in women is linked to the presence of other tumors in the anogenital region, probably related to everyday exposure to HPV due to anatomical proximity (both the cervix and the anal canal act as reservoirs that will favor mutual infection by proximity, regardless of its initial anatomical location). Thus, patients presenting with neoplasia in this region will have a considerably higher risk than those with a second neoplasia in the anogenital region14.
Risk factors for HPV infection include sex with uncircumcised men, a partner who has had many sexual partners, and first sexual contact at an early age, as well as patients who have had a solid organ transplant (kidney, liver, among others), are undergoing chemotherapy or radiotherapy (leukemia, lymphomas), use biologicals, are chronic steroid users, have diabetes, among others, which consequently follow immunosuppressive treatment. Specifically, several studies have reported rates of anal carcinoma up to 10 times higher than the general population in patients with kidney transplants15.
What is anal dysplasia?
AIN is defined as dysplastic cells in the anal canal. However, cytologically, abnormal squamous cells of the anus are classified as ASCUS, atypical squamous cells (high-grade squamous intraepithelial lesion cannot be ruled out), LSIL, and HSIL10. It has also been confirmed that lesions classified as low-grade dysplasia (LGD) can undergo spontaneous regression without any treatment (50-70%) or progress to high-grade dysplasia (HGD) (20%). Approximately 10% of patients with HGD may progress to epidermoid carcinoma within three years after detection of the lesion.
The American Society of Colon and Rectal Surgeons recommends using the term low-grade squamous intraepithelial lesions (LSIL). The term comprising Bowen’s disease, carcinoma in situ, AIN II, AIN III, moderate dysplasia, and HGD is high-grade squamous cell intraepithelial lesions (HSIL) and are considered cancer precursor lesions or ASCC16.
In a case report, Sha et al. found that 5.3% of IBD patients were HPV-positive with ASCUS. In this cohort, there were no cases of LSIL, HSIL, or ASCC17.
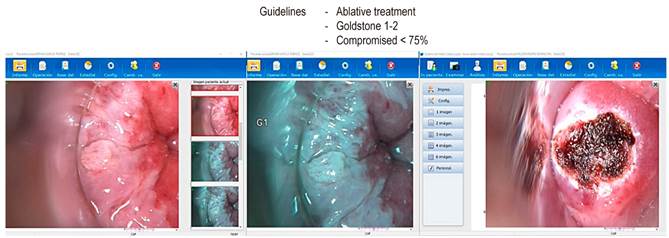
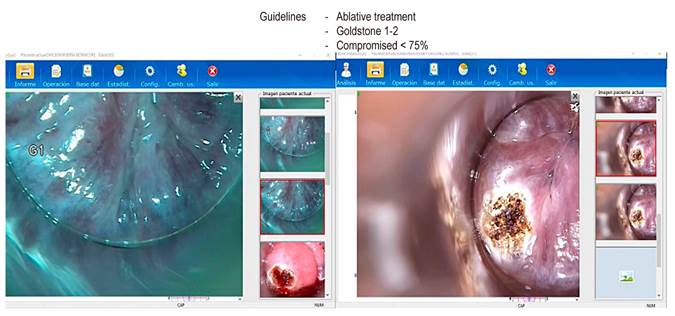
The gold standard for the diagnosis of AIN is HRA-guided anal biopsy, and it is currently classified into LGD and HGD based on the appearance of dysplastic cells that affect the epithelium from lesser to greater depth, respectively.
Both incidence of and mortality from anal cancer are becoming more common, with a 2.2% increase in new cases each year for the last decade and a 3.75% increase in mortality rates each year over the previous two decades10. Therefore, closer surveillance (3 to 4 months) should be considered because HSILs have been shown to recur within six months after treatment, and for having a high-grade lesion, the patient should be considered at higher risk of developing additional anal dysplasia than the general population18.
Why is inflammatory bowel disease oncogenic?
IBD is an inflammatory entity that favors alterations in cellular immunity. In a systematic review that included 11 studies of referral centers (1940 to 2005), the incidence of ASCC was 0.02 per 1000 patient-years in patients with Crohn’s disease (CD) and 0.009 per 1000 patient-years in patients with UC; that is, similar to that of the general population19.
It is known that in CD, there is a reduction in human defensins. These proteins are found in the immune system cells in the genital mucosa, helping against viral infections. They inhibit cutaneous and mucosal HPV, acting as a natural barrier against HPV.
Human α-defensins 1, 2, 3, and 5 have been shown to inhibit HPV in the skin and mucous membranes, which may account for an increased risk of HPV infection in IBD patients2. Most cases of ASCC that develop in patients with CD are diagnosed in the perianal manifestation of early onset in life with a long-term disease (>10 years)17,19.
Is the risk of cancer increased when associated with HPV and IBD?
The pathogenesis of IBD-related anal cancers is believed to be associated with disease-related mechanisms that include local and systemic changes, chronic inflammation, HPV infection, decreased function of defensins, specifically in CD, and drug-induced immunosuppression, which produces changes in the processes of proliferation, senescence, and cell death, as well as in DNA mutation and methylation.
There is a correlation between HPV infection and IBD: HPV is more common in patients with IBD, with a 3- to 5-fold increase in changes in cervical cytology in women with IBD compared to the general population20. There are several possible explanations for this correlation. HPV is likely a passenger virus that implants more frequently in IBD patients due to the weakened immune system, which is characteristic of these individuals. Another possibility is that the inflammation caused by IBD provides a hospitable environment for HPV to flourish. Interestingly, a PCR-based study demonstrated that all ASCC patients who were immunosuppressed as a result of IBD treatment tested positive for high-risk HPV variants21.
In a recent French study, 469 patients subject to consecutive procedures (median age: 54 years, 52% women), including 112 who received immunosuppressive therapies and 101 with IBD (70 with CD), underwent routine colonoscopies. HPV DNA in anal tissues was detected in 34% of subjects and high-risk HPV serotypes in 18%. Serotype 16 was the most prevalent genotype (seen in 7%), followed by 51, 52, and 39. High-risk serotypes were detected in a significantly higher proportion of samples from women (23.1%) than from men (12.8%) (p = 0.0035) and in a substantially higher proportion of patients with CD (30.0%) than without it (18.1%) (p = 0.005). Overall, 84/101 (83.2%) IBD patients were on immunosuppressive therapy at the time of the study (75.8% vs. 85.7% for UC and CD patients, respectively, p = 0.25). Of these, 30.8% had infections with high-risk strains, compared to 16.6% of unexposed patients (p = 0.15)22.
In a study of 26 IBD patients, half of whom were taking immunomodulators, 81% of patients were found to carry anal HPV (80% had ≥1 high-risk HPV type), and 42% of patients had abnormal anal cytology. All patients taking a thiopurine had >1 high-risk anal HPV detected. Among patients who underwent biopsies, 38% had LSIL and 15% had HSIL. Of the group with dysplasia (LSIL/HSIL), 43% were taking immunosuppressants17.
ASCC in patients with IBD has a poor prognosis, with a 5-year survival of 37%, according to the only systematic review available19. The general population’s 5-year survival rate after ASCC diagnosis exceeds 60%23. Therefore, IBD patients should be screened for HPV and, if positive, monitored for the development of cancer, specifically in patients with long-standing perianal disease19.
The mainstay of treatment for HPV anal dysplasia has traditionally been surgical excision. However, its effectiveness may be limited, with high morbidity and disastrous functional and anatomical sequelae, such as anal stricture or fecal incontinence. Therefore, the modern approach that has been established as a management standard at the Instituto de Coloproctología ICO S. A. S. is based on two approaches: HRA-directed ablative therapy and ablative medical therapy based on the use of 5-fluorouracil together with methotrexate in a scheme of 5 days of active application twice a day, spaced by nine days of rest for a total of 16 weeks.
HPV vaccines, such as Gardasil 4 or 9 and Cervarix, effectively reduce infection rates, but their effectiveness has not been studied in people with IBD.
Does anti-IBD treatment promote HPV infection?
The treatment of IBD, except for aminosalicylates, is based on immunomodulation (azathioprine, methotrexate, and 6-mercaptopurine) or immunosuppression with biological agents such as tumor necrosis factor blockers (anti-TNF) (infliximab, adalimumab, golimumab, and certolizumab pegol). These drugs impair cellular immunity and are associated with higher rates of viral, bacterial, and fungal infections, with increased risk in those using combination therapy, which may result in a higher rate of HPV-associated diseases, including genital warts, dysplasia, anogenital, and even oropharyngeal cancer.
In one cohort, ASCUS’s overall prevalence was 7% (8.8% in the IBD group vs. 2.6% in healthy controls). This is similar to the prevalence in other low-risk populations (3.9-10%) but lower than that of AIN reported in other high-risk populations (19.6-28%), such as those with genital dysplasia or a history of transplant17.
According to a recent meta-analysis, long-term exposure to immunosuppressive therapy in IBD could promote HPV-associated cervical dysplasia and cancer (odds ratio [OR] = 1.34, 95% confidence interval [CI]: 1.23-1.46)24. Another study documented that the relative risk of inducing cervical cancer was 1.65 for 5-aminosalicylic acid (5-ASA) and 3.45 for thiopurines (both with p-values >0.05)25.
However, there are controversial data, as expressed in an article in which no differences were found in cytological alterations and the presence of HPV in the anal canal between patients with CD and a control group of healthy people. On the contrary, in other retrospective case series, the authors concluded that anal squamous neoplastic lesions in IBD are associated with HPV infection, and ASCC seems to be associated with perianal CD11,26.
A prospective study of 230 IBD patients demonstrated a significant increase in viral warts in the group receiving AZA/6-MP compared to those not receiving immunosuppression (17.2% vs 3.3%, p = 0.004). Abnormal Pap tests in women with IBD compared to controls (42% vs. 7%, p = 0.001) and also in women with a history of exposure to immunosuppression back the recommendation of HPV vaccination and regular gynecological examinations in women11.
TNF is the cornerstone in multiple cellular processes, such as the regulation and maintenance of homeostasis of the immune system, inflammation, and host defense27-29. One article investigated patients using anti-TNF-α medications and found that 21.1% were positive for HPV infection in the genital region30. In recent years, there has been a resurgence of interest in the parallels between chronic inflammation and cancer, and it is not surprising that TNF-α has become the focus of this research.
Given its close development with inflammatory processes and its participation in the apoptosis signal, its significant role has been seen in the modulation of HPV infection since, in infected cells, it helps stop the replication and spread of the virus. Increased expression of TNF-α has been observed in HPV infection in both normal cervical tissues and cervical cancers, supporting the importance of TNF-α in the response to HPV and the subsequent carcinogenesis31.
TNF-α suppresses the expression of the E6 and E7 oncogenes at the translation level in human cells infected by HPV16 by binding to its type I receptor31,32, induces apoptosis in normal and infected cells by HPV16, and stimulates the inflammatory response by regulating cytokines and other inflammatory regulators. Therefore, TNF-α blockade may increase the risk of HPV reactivation and ultimately lead to host cell apoptosis. Screening and vaccinating patients receiving TNF-α antagonists is essential31-34.
The association between cervical dysplasia and TNF-α blockade therapy is well documented, given the latter’s fundamental role in the control of viral infection, including HPV. Therefore, therapeutic inhibition of TNF-α may increase the risk of HPV reactivation and cause cervical dysplasia and carcinoma, as noted in patients with rheumatoid arthritis and CD. Wadstroem et al. analyzed the risk of cervical dysplasia in patients with rheumatoid arthritis treated with anti-TNF. They found that they had an increased risk of high-grade cervical dysplasia (hazard ratio: 1.36 CI: 1.01 to 1. 82) and invasive cervical cancer (hazard ratio: 2.10, CI: 1.04 to 4.23) compared to women without biological treatment35.
Another study by Kane et al. included CD patients exposed to immunomodulators, including prednisone, purine analogs, methotrexate, and infliximab, who underwent Pap smears and compared their cytology over two years with those of a control population. Women exposed to immunosuppressive therapy for more than six continuous months were more likely to have abnormal cytology (p = 0.001) than the control population36. Nonetheless, upon closer examination of individual studies, the evidence regarding cervical abnormalities attributable to thiopurines and anti-TNF is still inconclusive37.
This opens the door to questioning whether the long-term use of anti-TNF blockers or immunosuppressants in patients with IBD may increase the risk of anal cancer, taking into account the close link with HPV clearance. However, it should be recognized that the CESAME cohort did not demonstrate an association between thiopurine use and anal cancer (either adenocarcinoma or ASCC) in IBD patients, although the crude risk in the subgroup of perianal CD patients exposed to thiopurines was of 0.42 per 1000 patient-years. Furthermore, the TREAT and ENCORE registries have not demonstrated an excess of anal cancers in patients treated with infliximab38-40.
The consensus statement of the European Crohn’s and Colitis Organization (ECCO) only recommends vaccination in women suffering from IBD to prevent cervical cancer. However, unfortunately, there are no specific recommendations for HPV prophylaxis to prevent ASCC in patients with IBD41,42.
Given the well-established similarities between cervical and anal HPV-related diseases, recent CDC guidelines have recommended HPV vaccination for children of both sexes at age 11 or 12 and for men and women at high risk of AIN or CIN or any previously unvaccinated person under 26 years of age43.
In studies of MSM who are HIV-negative, the sensitivity of anal cytology reported in the literature varies, ranging between 47% and 70% for the detection of AIN of any grade. HSIL anal cytology correlates well with high-grade AIN (i.e., AIN II or III), so direct anal examination and tissue biopsy are recommended after any abnormal anal cytology result44.
If abnormal cytology is detected on anal cytology examination, the next step in the management of AIN is HRA to attempt to localize the source of atypical cells. HRA involves examining the squamocolumnar junction, anal canal, and perianal skin under magnification using a colposcope. During anoscopy, a lidocaine-lubricated anoscope is inserted through the anus. Then a swab soaked in a 3-5% acetic acid solution is inserted into the anal canal while the anoscope is removed for one minute. Acetic acid causes an “acetowhite change” in areas of the abnormal transitional epithelium; the mucosa is carefully inspected for changes characteristic of AIN, including flat or slightly raised areas of thickened mucosa with or without abnormalities in the vascular pattern. Lugol’s iodine is then applied similarly, but in this case, the lesions do not stain with iodine (negative for Lugol’s stain) because iodine is glycophilic, and the dysplastic tissues lack glycogen and appear thick mustard in color. Any suspicious Lugol’s iodine-negative lesion, including condylomas, atypical surface configurations, stippling, mosaicism, or atypical vessels, is biopsied under direct visualization (Figures 2, 3, and 4).
HRA is considered superior to standard anoscopy, as demonstrated by Camus et al., who reported that in a population of 102 patients (68% men, 57.3% HIV positive; mean: 1.6 lesions), only 38.7% (65/168) of all lesions observed with HRA were visible with standard anoscopy45.
Although HRA is generally considered safe for patients and is not difficult for clinicians to perform, considerable training time is required to recognize anal lesions, especially those that may have a subtle appearance. Due to the limited number of patients with atypical findings associated with AIN in the general population, HRA is ideally performed in specialized centers46.
Strategies to reduce HPV infection
Behavioral factors also play a role in preventing HPV infection. Stopping smoking, in addition to promoting and educating about safer sexual practices, can reduce the prevalence of people infected with HPV and, in turn, could reduce the incidence of anal cancer risk caused by HPV in patients with IBD.
Anal cytology performed by trained personnel detects precancerous and intraepithelial lesions that could eventually lead to invasive anal carcinoma. As a screening tool, it is as sensitive for detecting anal cytological abnormalities in high-risk patients as it is for cervical cancer and contributes to the careful selection of patients for HRA.
AIN can be diagnosed with screening strategies such as anal cytology and HRA, thus avoiding progression to anal cancer, with an acceptable cost and low morbidity.
The risk of non-fistula-related ASCC in UC patients is the same as in the general population. It does not justify an anal cancer screening program in IBD patients who are not at specific high risk due to associated HIV infection or a personal history of anogenital condyloma.
Conclusion
Our study suggests that anal squamous neoplasia in IBD is associated with HPV infection and that ASCC appears to be associated with perianal CD. In addition to careful perianal examination, anal HPV screening could be considered in patients with IBD. Still, it seems too early to provide clear recommendations for screening a specific subset of patients. A prospective study is needed to confirm these findings. Although based on low-level evidence from uncontrolled studies, annual perianal examination with or without anal cytology could be considered in IBD patients with long-standing perianal fistulizing disease, anal stricture, or known HPV infection.
Additionally, IBD patients with other risk factors for HPV infection, such as a history of receptive anal intercourse, MSM, history of sexually transmitted diseases (including HIV), multiple sexual partners, and history of anogenital cancer (cervical, vulvar or vaginal) should also be screened to prevent anal cancer11.
Our study demonstrates that patients with IBD behave as a high-risk group for developing premalignant lesions in association with HPV, such as dysplasia in 35.5% and condylomas in 16.1%. Vaccination reduces the rate of high-grade anal intraepithelial neoplasia related to high-risk HPV strains by 75%. In patients with IBD, the ECCO recommends routine prophylactic HPV vaccination for both women and men, according to national guidelines47.
The persistent inflammatory state, the use of steroids, and anti-TNF play a vital role in decreasing HPV viral clearance, so a more exhaustive study should be carried out in patients with IBD, establishing the frequency of lesions and the viral genetic profile that could be related to the development of anal cancer.
HRA is a cost-effective, painless, and affordable method that, in expert hands, can efficiently diagnose and treat premalignant lesions and decrease the incidence of anal cancer in patients with IBD.
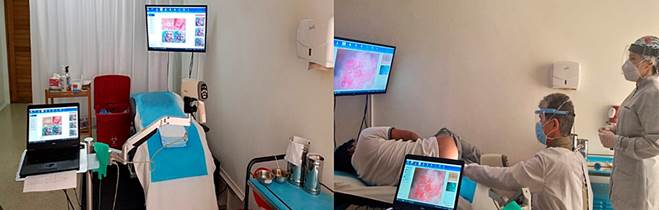
To date, through ICO Seguro, we are the first program designed and implemented in Colombia that comprehensively guarantees the education of health personnel and patients and the detection, treatment, and monitoring of populations at risk of anal cancer (Figure 5).

Source: Authors’ archive.
Figure 5 The human team of the Instituto de Coloproctología ICO specialized in high-resolution anoscopy.
Nevertheless, in the end, there are more questions than answers: Are there specificities in the prevalence and serotype distribution of HPV infection in patients with IBD compatible with other established risk factors for HPV infection (smoking, sexual practices)? Does immunosuppression increase the risk of HPV-related ASCC and intraepithelial precursor lesions in patients with IBD and HPV infection? Is the excess risk of non-fistula-related ASCC in patients with CD associated, at least in part, with local or systemic chronic inflammation? What are the optimal frequency and surveillance modes between digital examination, cytology, and HRA?











 texto en
texto en