Introduction
The most accepted definition of quality of life in the academic and scientific community is established by the World Health Organization (WHO), which refers to an individual’s well-being1. Physical and mental health have a marked effect on the quality of life, which is why the concept of health-related quality of life (HRQoL) has been introduced2. It has been estimated in patients with cirrhosis and other chronic diseases using generic and specific scales3-9, observing good reproducibility and consistency.
Sexual dysfunction is frequent in people with cirrhosis; the most probable cause is suppression of the hypothalamic-pituitary-gonadal axis10. The characterization of the sexual function of patients with chronic diseases through proper tools allows for assessing the global impact of the disease on these subjects11-17.
Female sex has been identified as a predictor of worse quality of life in patients with chronic liver disease in various studies18,19. The determinants of this difference between the sexes have not been evaluated in detail. The relationship between sexual function and quality of life has been described in other chronic diseases16,17, so it would be plausible to speculate that the former could explain part of the differences in HRQoL between men and women with liver cirrhosis.
The objective of this study is to determine the relationship between quality of life and sexual function in women with liver cirrhosis treated at a hepatology center in Cartagena.
Materials and methods
A cross-sectional analytical observational study was carried out. The inclusion criteria were female, unequivocal cirrhosis diagnosis by clinical, imaging, analytical, or elastographic criteria, and aged between 18 and 69. A trained interviewer administered the SF-36 V2 and the FSFI-6 surveys during the same interview. Complementary information was collected from medical records. Laboratory test values were only considered if performed in the last three months.
Exclusion criteria
Limitations in answering the instruments verified by the researchers during the clinical evaluation. Patients with a previous diagnosis of diseases that limit physical or mental capacity, such as disability, sequelae of neurological disease, or moderate to severe cognitive impairment at the discretion of the researchers.
Instruments
The SF-36 V2 is a 36-item questionnaire that evaluates eight areas of HRQoL and generates two indicators that summarize the physical (PCS) and mental (MCS) components of the scale, namely, physical functioning (PF), role-physical (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role-emotional (RE), mental health (MH); with scores per dimension ranging from 0 to 100. The higher the score, the better the quality of life20. Qualitimetric Inc. granted permission to use the SF-36 V2 survey in Spanish, endorsed by the International Quality of Life Assessment Project Approach (IQOLA) for administration in Colombia21.
The 6-item Female Sexual Function Index (FSFI-6) is a tool developed to assess female sexual function; it comprises six domains: desire, arousal, lubrication, orgasm, satisfaction, and pain. Its score ranges from 2 to 30; lower scores indicate poor sexual function. A score ≤ 19 reports a sensitivity of 93% and specificity of 94% for sexual dysfunction22.
Statistical analysis
Quantitative and categorical variables were described through means (SD) and percentages as appropriate for numerical and categorical variables. To estimate the relationship between quality of life and sexual function, we used a simple linear regression analysis in which the PCS score of the SF36-V2 scale was the dependent variable, and the global score of the FSFI-6 scale was the independent variable. The same analysis was performed using the MCS score as the dependent variable. The factors associated with female sexual function were identified using a linear regression analysis. The dependent variable was the FSFI-6 scale score, and the independent variables were the sociodemographic and clinical variables. To identify factors related to quality of life, a simple linear regression analysis was performed in which the PCS and MCS score of the SF36-V2 scale were the dependent variables and the sociodemographic and clinical variables (etiology, Child-Pugh score, history of decompensation, type of decompensation, presence of esophageal varices, comorbidities, age at menopause, albumin value, total bilirubin, aminotransferases, platelets, international normalized ratio [INR], creatinine) the independent ones. Those variables with a statistically significant association in the simple linear regression were included in a multivariate analysis. A p-value less than 0.05 is the criterion for statistical significance in all cases. The software used was SPSS version 15.
Results
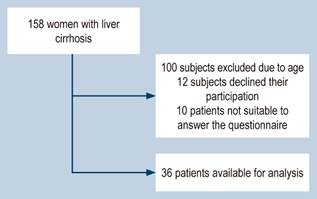
Thirty-six patients were available for analysis. Figure 1 shows the flow of patient inclusion into the study. The mean age was 61 years (95% confidence interval [CI]: 58-64; standard deviation [SD]: 7.7). The mean PCS value was 58 (95% CI: 49-66; SD: 23), and the mean MCS value was 56 (95% CI: 49-63; SD: 18). The mean FSFI-6 score was 10 (95% CI: 6.8-13; SD: 8.5). 80.5% of the patients had sexual dysfunction using a cut-off point in the FSFI-6 ≤ 19, and 83.3% of the women were postmenopausal. The mean menopausal age was 44 years (95% CI: 39-48; SD: 12.35). 69.4% of the sample was classified in Category A of the Child-Pugh score, while 30.6% was in Category B.
The most common cause of liver cirrhosis in this group of women was non-alcoholic steatohepatitis (41.7%), followed by autoimmune etiology (19.4%), viral etiology (16.7%), and other causes (primary sclerosing cholangitis, alcoholic liver disease, cryptogenic, among others), which represented 22.2%. There were esophageal varices in 61.1% (small: 19.4%, medium: 16.7%, and large: 25%) and a history of variceal bleeding in 27.8%. The history of decompensation had a frequency of 44.4%, and a history of ascites was found in 19.4% of the total sample. Obesity was present in 19% of the women in the study; the mean body mass index (BMI) was 27.54 kg/m2 (CI: 25.8-29.2; SD: 4.72). A more detailed description of the patient’s baseline characteristics can be found in Table 1.
Table 1 Demographic and clinical characteristics
ALT: alanine aminotransferase; AST: aspartate aminotransferase; BB: β-blocker; BO: bodily pain; BMI: body mass index; FSFI-6: 6-item female sexual function index; GH: general health; HBP: high blood pressure; INR: international normalized ratio; MH: mental health; MCS: mental component summary; MRA: mineralocorticoid receptor antagonist; NASH: non-alcoholic steatohepatitis; PF: physical functioning; PCS: physical component summary; RE: role-emotional; RP: role-physical; SF: social functioning; VT: vitality. Prepared by the authors.
Factors associated with sexual function in cirrhotic women
When estimating the relationship between quality of life and sexual function through a linear regression analysis, the FSFI-6 score positively relates to PCS (B coefficient: 0.365; p = 0.029). As the FSFI-6 values increase, so will the PCS values. This relationship was not observed with the MCS (B coefficient: 0.219; p = 0.199) (Table 2).
Table 2 Relationship of quality of life with sexual function. Univariate linear regression analysis. Dependent variable, physical component summary, and mental component summary of the SF36v2 scale
| Variable | B | 95% CI | p | |
|---|---|---|---|---|
| Physical component summary | FSFI-6 | 0.36 | 0.10-1.8 | 0.029 |
| Mental component summary | FSFI-6 | 0.219 | (-0.246) - (1.13) | 0.199 |
FSFI-6: 6-item female sexual function index. Prepared by the authors.
The linear regression analysis of the factors associated with sexual function point to age (B coefficient: -0.525; p = 0.001) and menopause (B coefficient: -0.387; CI: 0.020) as predictors of deterioration in sexual function of this population. However, this relationship was not found in some variables that could have been relevant, such as the Child-Pugh score (B coefficient: -0.291; p = 0.085), the history of decompensations (B coefficient: -0.276; p = 0.103), BMI (B coefficient: -0.203; p = 0.235), obesity (B coefficient: -0.148; p = 0.389), or diabetes (B coefficient: -0.091; p = 0.596) (Table 3).
Table 3 Factors related to sexual function (FSFI-6). Univariate linear regression analysis. Dependent variable, FSFI-6 scale
| Variable | B | 95% CI | p |
|---|---|---|---|
| Age | -0.525 | (-0.75) - (-0.209) | 0.001 |
| SEL | 0.258 | (-0.751) - (5.70) | 0.128 |
| Weight | -0.234 | (-0.356) - (0.065) | 0.17 |
| BMI | -0.203 | (-0.983) - (0.249) | 0.235 |
| Obesity | -0.148 | (-10.6) - (4.25) | 0.389 |
| Dyslipidemia | -0.073 | (-7.83) - (5.10) | 0.67 |
| HBP | -0.096 | (-8.0) - (4.55) | 0.57 |
| Diabetes | -0.091 | (-10.0) - (5.8) | 0.59 |
| Child-Pugh score | -0.291 | (-11.6) - (0.78) | 0.085 |
| Decompensation | -0.276 | (-10.5) - (1.01) | 0.103 |
| Variceal bleeding | -0.028 | (-7.19) - (6.13) | 0.872 |
| Ascites | -0.271 | (-13.1) - (1.39) | 0.11 |
| Total bilirubin | -0.9 | (-3.0) - (1.7) | 0.6 |
| AST | -0.183 | (-0.144) - (0.043) | 0.284 |
| ALT | -0.03 | (-0.07) - (0.064) | 0.861 |
| Albumin | 0.189 | (-2.60) - (9.0) | 0.268 |
| Creatinine | -0.089 | (-10.6) - (6.35) | 0.613 |
| Menopause | -0.387 | (-16.2) - (-1.50) | 0.02 |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; BMI: body mass index; HBP: high blood pressure; SEL: socio-economic level. Prepared by the authors.
Factors affecting health-related quality of life
The simple linear regression analysis identified that the factors associated with HRQoL, taking PCS as the dependent variable, were BMI (B coefficient: -0.388; p = 0.019), creatinine (B coefficient: -0.376; p = 0.026), the Child-Pugh score (B coefficient: -0.733; p = 0.000), and other variables associated with this scale, such as decompensations (B coefficient: -2.7, p = 0.009), ascites (B coefficient: - 0.412, p = 0.012), and albumin (B coefficient: 0.437, p = 0.008); sexual function through the FSFI-6 scale showed an association with HRQoL (B coefficient: -0.365; p = 0.029). In the multivariate analysis, only BMI (B coefficient: -0.291; p = 0.008) and the Child-Pugh score were identified as factors associated with HRQoL. Other variables such as diabetes (B coefficient: -0.0004; p = 0.98), esophageal varices (B coefficient: 0.053; p = 0.75), history of variceal bleeding (B coefficient: -0.13; p = 0.45), and menopause (B coefficient: -0.101; p = 0.55) did not have a relationship in the simple linear regression.
The factors related to HRQoL in the simple linear regression analysis taking MCS as the dependent variable were the Child-Pugh score (B coefficient: -0.559; p = 0.00), as well as related variables (decompensation, albumin, ascites) and BMI (B coefficient: -0.396; p = 0.017). In the multivariate analysis, the relationship with the Child-Pugh score (B coefficient: -0.49; p = 0.0001) and BMI (B coefficient: -0.49; p = 0.045) was maintained. Diabetes (B coefficient: -0.113; p = 0.513), esophageal varices (B coefficient: 0.132; p = 0.442), history of variceal bleeding (B coefficient: -0.051; p = 0.76), creatinine (B coefficient: -0.209; p = 0.229), and menopause (B coefficient: 0.068; p = 0.694) showed no relationship with MCS (Tables 4 and 5).
Table 4 Factors associated with quality of life (PCS). Univariate linear regression analysis. Dependent variable, physical component summary of the SF36v2 scale
ALT: alanine aminotransferase. AST: aspartate aminotransferase; BMI: body mass index; HBP: high blood pressure; SEL: socio-economic level. Prepared by the authors.
Table 5 Factors associated with quality of life (MCS). Univariate linear regression analysis. Dependent variable, mental component summary of the SF36v2 scale
ALT: alanine aminotransferase. AST: aspartate aminotransferase; BMI: body mass index; HBP: high blood pressure; SEL: socio-economic level. Prepared by the authors.
Discussion
A linear relationship between sexual function and HRQoL was observed in women with liver cirrhosis. The PCS values increased as those of the FSFI-6 scale also increased, and this association was statistically significant in the univariate analysis. This relationship has also been noted in women with cervical cancer and chronic kidney disease16,23.
The average FSFI-6 was low (10.8 points) in this population; in fact, 80% of the patients presented with sexual dysfunction (FSFI-6 ≤ 19) according to the cut-off points reported in the literature22. Although this finding could be explained by the old age of the patients (61 years) and the high percentage of menopause in this cohort (83%), the FSFI-6 scores were low in pre- and postmenopausal women (19 vs. 9.3 points). From a biological point of view, the impairment of sexual function in cirrhosis is multifactorial. The autonomic dysfunction and changes in the female genital tissue caused by ovarian atrophy and premature menopause in these patients can affect desire and orgasmic response, not to mention psychiatric and affective disorders such as depression, which can also play an essential role in female sexual function10.
Age and menopause were independent predictors of sexual function in patients with cirrhosis. Other studies conducted in different geographic regions in the general population consistently support these results. Research carried out in Istanbul on 1009 women found that menopause increased the risk of sexual dysfunction by 84% (p = 0.046), and this risk could be up to 60% higher when comparing women aged 60-64 years versus those aged 20-29 (p = 0.000)24.
The relationship between menopause and sexual dysfunction has been widely described, and this can be explained by multiple factors such as pain during sexual intercourse, genitourinary syndrome, and vaginal dryness, among others. A study published in 2017, which included 405 postmenopausal women, described a positive relationship between the FSFI score and quality of life questionnaires in all its domains25, and this subgroup of patients was identified as susceptible to additional interventions that specifically improve their sexual function and quality of life.
A study conducted in Turkey, which included 282 healthy women, of which about 40% were over 40 years of age, found a prevalence of sexual dysfunction of 53.2% using the FSFI scale, and the risk was higher in older patients with urinary problems26. In our cohort, the prevalence of dysfunction was 80%, suggesting a possible role of cirrhosis as a determining factor in female sexual function.
Chronic liver disease and not necessarily cirrhosis also influences female sexual function. A case-control study published in 2014, which included 337 women, compared a group of sexually active women with chronic hepatitis C without liver cirrhosis with another group of sexually active healthy women. In the chronic hepatitis C group, the scores of FSFI were significantly lower, and sexual dysfunction was more frequent (79% vs 21%, p < 0.05)27.
There are few studies about sexual dysfunction in women with liver cirrhosis. A study published in 1989 found that of 150 women with non-alcoholic liver disease, sexual desire decreased in 33%, difficulty achieving arousal was observed in 18%, orgasm was not experienced in 25%, the frequency of sexual intercourse decreased since the onset of the disease by 27%, and dyspareunia occurred in 21%28.
Only the Child-Pugh score and BMI were identified as independent predictors of HRQoL in the multivariate analysis in both the PCS and the MCS, which is consistent with previous studies in Colombia in which the Child-Pugh score, female sex, viral etiology, and albumin were identified as determinants18. Similarly, in a prospective cohort of 92 cirrhotic men and women, the Child-Pugh score and BMI were identified as factors associated with HRQoL29.
The association of BMI with HRQoL is not limited to patients with cirrhosis. In a work published in 2018, which included 10,133 subjects, of which 71.7% were women, there was a significant association between BMI and HRQoL for the physical and mental components30. Additionally, an investigation of obese subjects published in 2014 in which 25 men and 70 women participated revealed the association between BMI and sexual function, with an inverse relationship between sexual function and BMI/waist circumference31.
In 2013, Morotti published a study that included 90 women and found less vascularization of the clitoris in obese women compared to overweight or slim women; sexuality surveys reported higher scores in thin women compared to overweight and obese women. The percentage of anorgasmic women was higher in obese patients than in thin patients (23% versus 6%), and in the questionnaires that evaluated depressive symptoms, higher scores were found in obese patients. Furthermore, there was greater dissatisfaction with their figure and body32. These data indicate that weight has an essential association with quality of life and sexual function in women, and this effect remains and could even be more significant in women with chronic diseases such as cirrhosis. Still, in our study, BMI was a predictor of HRQoL but not of sexual function.
History of decompensation, ascites, and albumin were related to HRQoL for PCS and MCS. These findings likely occur because they are variables associated with the severity of cirrhosis and, therefore, it is common for patients with more significant alterations to have more advanced disease.
Despite the sample size, the consistency of our findings with those reported in other cohorts of patients with cirrhosis worldwide supports the robustness of our results. The inclusion of patients in the study was limited by the age range in which the FSFI survey was validated for Colombia (18-69 years)33, the silent nature of the disease, and the lack of specific screening programs for chronic liver disease in our country, which has caused patients to be diagnosed at late ages.
Female sexual dysfunction is not actively evaluated in the consultation of patients with liver cirrhosis despite its high prevalence in this population. A comprehensive approach to the treatment of these patients should include the evaluation of the sexual sphere and BMI to identify patients who would benefit from an assessment by specialists in the area to make interventions that improve HRQoL in this population.
Conclusions
In women with liver cirrhosis, sexual function is a determinant of HRQoL, and the factors that were associated with sexual dysfunction were age and history of menopause. The progression of the disease and weight are related to the deterioration of HRQoL. The conduct of new studies and the implementation of multidisciplinary programs that affect these determinants of quality of life and sexual function are necessary for the comprehensive management of women with this pathology.











 texto em
texto em