Introduction
Annually, approximately 600,000 people are diagnosed with esophageal cancer worldwide1. More than half of patients present with inoperable disease at the time of diagnosis due to metastases or a poor medical condition. Dysphagia is a common symptom that dramatically impacts the patient’s quality of life2. Although brachytherapy is superior in achieving long-term relief of dysphagia, esophageal stenting induces a more rapid resolution3. Therefore, it is currently accepted that the placement of an esophageal prosthesis is indicated mainly in patients with short-term survival4.
The first description of the clinical use of esophageal prostheses is attributed to Celestin, who collected previous experiences and results with the use of plastic esophageal prostheses to treat malignant dysphagia5. In Colombia, the first publication on the clinical use of plastic and rigid prostheses in the esophagus dates back 35 years by the group of the National Cancer Institute6. The first publication in the field using self-expanding metal prostheses is from 20 years ago, with the advantage of being locally built prostheses7. Currently, esophageal stenting is used for a wide variety of esophageal diseases. Various stent designs with different characteristics are available for clinical use. The attributes of esophageal stents vary depending on mechanical properties, such as the material (metal, plastic, or biodegradable), the radial and axial forces acting on the lumen of the esophagus, and the type and design of the cover (partial, total, or uncovered) surrounding the mesh of the prosthesis (silicone, polyurethane, among others). The implications of different stent characteristics on clinical outcomes have not been fully elucidated due to a lack of results from randomized, controlled clinical studies8.
While esophageal prostheses can effectively restore luminal patency, they are not complication-free. Minor complications of esophageal stenting include chest pain, acid reflux, stent obstruction from tissue growth or poor diet, recurrent dysphagia, and stent migration. Moreover, the major adverse effects are bleeding, aspiration pneumonia, and perforation. These adverse effects may require repeating the endoscopy with possible stent removal9. Our experience with esophageal prostheses has been previously described, and we demonstrated that esophageal stents can improve dysphagia but do so imperfectly, with high complication rates (29.4% of patients experienced at least one minor complication)10,11. Several prostheses have been designed to minimize these risks in recent decades, with different materials, shapes, sizes, biodegradability, types, extent of coating, and anti-reflux, anti-migration, or even radioactive characteristics12-16. Still, whether these technical developments have positively impacted clinical outcomes remains uncertain.
The profile of patients selected for treatment with stents has changed with variations in the treatment of esophageal cancer (chemoradiotherapy). Patients who underwent esophageal stenting for the management of malignant dysphagia over the past 25 years were analyzed for dysphagia recurrence in two periods, describing other related adverse events with stents and survival in these two periods.
Materials and methods
Study population
We included patients who underwent placement of a self-expanding metal esophageal prosthesis between January 1997 and May 2022 with palliative intent for malignant dysphagia due to esophageal or cardia obstruction, treated in four quaternary care institutions in Medellín attending cancer cases. Patients with a malignant stricture at the anastomosis after partial esophagectomy or gastrectomy with or without concomitant fistula were also included.
Eligible subjects were identified from the institutions’ database, reviewing endoscopy records and clinical studies from the four oncology institutions. Patients who received a self-expanding plastic stent were excluded.
Placement of the esophageal prosthesis
Different stents were placed during the study according to their historical evolution (steel, Z stents, nitinol). Still, the analysis was limited to evaluating whether the prosthesis was totally or partially covered due to inconsistencies in the description in the endoscopic report. The location, grade of stricture, and path were determined essentially by endoscopy and esophagram. The stent chosen depended on the center’s availability and the doctor’s discretion. Stenting was performed with the patient under conscious sedation. The lesion was inspected and explored with a standard endoscope when allowed by the diameter. If the stricture could not be crossed, the option was NOT to dilate and pass a guidewire. The esophageal location of the tumor was defined using the distance of the incisors from the upper margin of the tumor. It was subdivided into proximal (up to 22 cm), middle (22 to 28 cm), and distal (below 28 cm).
The stents were inserted over a guidewire and most frequently placed with no fluoroscopic but endoscopic control. The length of the esophageal prosthesis was determined considering the size of the stricture plus 4 cm, a minimum of 2 cm at each end above and below the tumor.
The fluoroscopy offers the option of having the path and length of the stricture better characterized to choose the size of the stent, but this choice was at the operator’s discretion.
Generally, patients received a liquid diet the same day after the procedure. They also received detailed feeding instructions at the time of hospital discharge.
Objectives and data collection
The primary objective was to determine the clinical efficacy and safety of esophageal prosthesis placement regarding relief and recurrence of dysphagia and diachronically evaluate occurrence changes. The secondary objective was to identify risk factors for recurrent dysphagia and stent-related adverse events, clinical and technical success rate, dysphagia improvement, and survival.
The patient’s medical records and endoscopy reports were reviewed, extracting the following data: age, sex, dysphagia score, previous chemotherapy or radiotherapy, location of stricture, histology, date of stenting, type of stent (covered, uncovered, partial), and the use of dilation. Most data were retrospective, although data from prospective studies were also included from the institutions’ electronic medical records and the online form (Drive).
Dysphagia was scored according to the Ogilvie scale17. The following outcome parameters were collected: technical success, complications, recurrent dysphagia, and survival. Technical success was defined as adequate deployment and placement of the stent in the intended position. Repositioning was allowed during the same procedure; however, if a second endoscopy with repositioning of the prosthesis was indicated, it was considered a technical failure.
Recurrent dysphagia was defined as stent migration, growth or overgrowth, occlusion of the alimentary canal, or other stent-related causes confirmed by endoscopy. Major complications involved severe or life-threatening complications, including perforation, hemorrhage, pneumonia, fever, fistula, or pressure necrosis. Minor complications included substernal pain and reflux symptoms. Time to major or minor complications was defined as the number of days from prosthesis placement to the first adverse event. Both complications were included if a minor and a major complication occurred in the same patient.
For evaluation purposes, the study was grouped into two 12-year periods, according to the date of stenting: Group 1 (January 1997 to December 2009) and Group 2 (January 2010 to May 2022). Data were collected until May 2022 to have a minimum follow-up of six months for the latest patients. The distribution into these two groups (first and second half of the series) considered the changes in the therapy approach (chemotherapy and neoadjuvant radiotherapy) given to esophageal cancer in the last ten years.
Statistical analysis
The occurrence of recurrent dysphagia, major and minor complications, and survival were evaluated using the Kaplan-Meier estimate and compared between the two consecutive periods using the log-rank test. Patients were censored at the time of death, after removal of the prosthesis, or at the end of follow-up. Univariate Cox regression analysis was performed to evaluate the association between multiple covariates, including time to prosthesis placement (in years) and the occurrence of recurrent dysphagia, subdivided into migration, tumor or tissue growth, and major and minor complications. Other covariates were age, sex, previous radiation or chemotherapies, fistula presence, stricture location, histology, extrinsic compression, and anterior dilation.
The statistical program SPSS version 22.0 (Chicago, Illinois, United States) was used for data analysis. P-values of 0.05 were considered the limit of statistical significance.
Ethical considerations
All procedures conformed to the ethical standards of the committee responsible for human experimentation (institutional and national) and to the 1964 Helsinki Declaration and later versions. The confidentiality of data was protected. The authors state that this article does not contain personal information that could identify patients. Data already analyzed from two studies were compared, which at the time were obtained from a secondary source without any intervention on the patients, so informed consent is not required.
Results
Characteristics of the patients
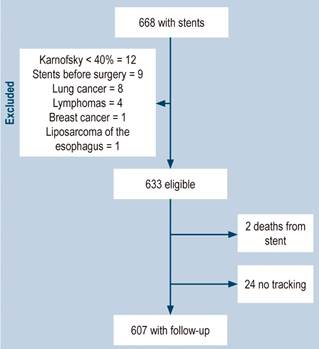
A total of 668 patients who underwent self-expanding metal esophageal stenting for malignant dysphagia between September 1997 and April 2022 were identified. The flow diagram of the study with the excluded patients is presented in Figure 1.
The calculations were based on 607 patients for whom the most information was available when reviewing the medical records. The clinical characteristics of the patients were discriminated into two temporary care groups: Group 1 (n = 289) with stents placed between September 1997 and December 2009, and Group 2 (n = 318) between January 2010 and May 2022. No differences were found in the median age or sex distribution in both groups (Table 1).
Table 1 Characteristics of patients with esophageal prosthesis due to malignant obstruction
| Characteristic | Group 1 (1997-2009) n = 289 (%) | Group 2 (2010-2022) n = 318 (%) | Total n = 607 (%) | p | |
|---|---|---|---|---|---|
| Age | 65.6 ± 11.7 | 68.2 ± 11.1 | 66.8 ± 10.2 | ||
| Sex | Male | 187 (64.7) | 212 (66.7) | 399 (65.7) | 0.611 |
| Female | 102 (35.3) | 106 (33.3) | 208 (34.3) | ||
| Location | Upper-middle esophagus | 87 (30.1) | 70 (22.0) | 157 (25.9) | 0.021 |
| Distal esophagus-cardias | 179 (61.9) | 222 (69.8) | 401 (66.0) | ||
| Relapse | 23 (8.0) | 26 (8.2) | 49 (8.1) | ||
| Fistula | Mediastinal | 10 (3.5) | 12 (3.8) | 22 (3.6) | 0.965 |
| Esophageal-respiratory | 13 (4.5) | 16 (5.0) | 29 (4.8) | ||
| No fistula | 266 (92.0) | 290 (91.2) | 566 (93.3) | ||
| Histology | Squamous cell | 116 (40.1) | 118 (37.1) | 234 (38.6) | 0.323 |
| Adenocarcinoma | 158 (54.7) | 190 (59.7) | 348 (57.3) | ||
| Other | 6 (2.1) | 10 (3.2) | 16 (2.6) | ||
| Unknown | 9 (3.1) | 0 | 9 (1.5) | ||
| Differentiation | Grade 2 | 15 (5.2) | 18 (5.7) | 33 (5.4) | 0.268 |
| Grade 3 | 181 (62.6) | 206 (64.8) | 387 (63.8) | ||
| Grade 4 | 74 (25.6) | 84 (26.4) | 158 (27.0) | ||
| Unknown | 19 (6.6) | 10 (3.1) | 29 (4.8) | ||
| Neoadjuvant | None | 167 (57.8) | 134 (41.6) | 301 (49.6) | < 0.001 |
| Chemotherapy | 50 (17.3) | 90 (28.3) | 140 (23.1) | ||
| Radiotherapy | 25 (8.7) | 34 (10.7) | 59 (9.7) | ||
| Chemo- + radiotherapy | 38 (13.1) | 52 (17.3) | 90 (14.8) | ||
| Unknown | 9 (3.1) | 8 (1.1) | 17 (2.8) | ||
| Fluoroscopy | Yes | 71 (24.6) | 48 (15.1) | 119 (19.6) | 0.006 |
| No | 218 (75.4) | 270 (84.9) | 488 (80.4) | ||
| Dilatation | Yes | 65 (22.5) | 43 (13.5) | 108 (17.9) | 0.002 |
| No | 224 (77.5) | 275 (86.5) | 499 (82.1) | ||
| Stent type | Fully covered | 192 (66.4) | 252 (79.2) | 444 (73.1) | 0.001 |
| Partially covered | 62 (21.5) | 48 (15.1) | 110 (18.2) | ||
| Unknown | 35 (12.1) | 18 (5.7) | 53 (8.7) | ||
| Success | Technical | 234/246 (95.1) | 280/286 (97.9) | 514/532 (97.0) | 0.95 |
| Clinical | 209/246 (84.9) | 252/286 (88.0) | 461/532 (87.0) | ||
| Unknown | 43 (14.9) | 32 (10.1) | 75 (12.0) | ||
Prepared by the authors.
All patients had dysphagia ≥ 2 before prosthesis placement. In the second period (Group 2), 56.3% of patients had been pretreated with chemotherapy or radiotherapy, compared to 39.1% in the previous period (p < 0.001). In 49 patients, stenting was indicated for residual or recurrent malignant obstructive disease. The proportion of patients with the more distally located disease (distal esophagus/cardias) increased from 61.9% to 69.8%, with a significant difference (p = 0.021). A fully covered stent was inserted 73.1% of the time. The proportion of completely covered prostheses increased in the second period from 66.4% to 79.2% (p = 0.01).
Technical aspects and improvement of symptoms
Placement of endoscopic esophageal stents was technically successful in 310 of 318 patients (97%). Technical failure occurred due to incorrect stenting (n = 6) and insufficient stent deployment (n = 2). No differences in technical success between the two study periods were observed (p = 0.95). However, it is evident that in the second period of the study, fluoroscopy assistance was used in a lower proportion (24.6% vs. 15.1%, p = 0.002). Dysphagia scores improved from a median of Grade 3 to 0 (p < 0.001) four weeks after stenting.
Fistulas in the esophagus
We found an esophageal fistula before stenting in 51 patients (6.7%), an esophageal-respiratory fistula in 29 (4.8%), and a mediastinal fistula in 22 (3.6%) (Table 1). In all these patients, the dysphagia score improved (2.81 vs. 1.3, p < 0.001). Median survival after stenting of fistulas was 78.1 days (range: 41-232 days).
Recurrent dysphagia
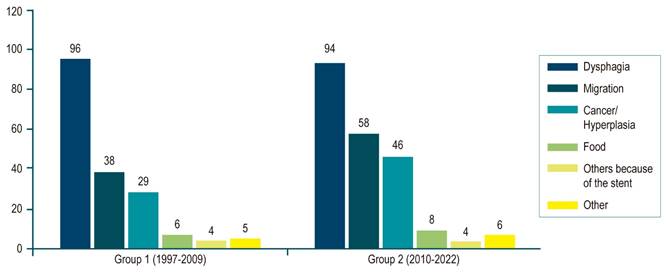
Recurrence of dysphagia occurred in 190 of 607 patients (31.3%) and was related to stent migration (96 patients; 15.8%) with internal or excessive growth of tumor or hyperplastic tissue (75 patients; 12.3%), occlusion of the alimentary tract (14 patients; 2.3%), other stent-related causes (8 patients; 1.3%), and other non-stent-related causes (13 patients; 2.1%) (Figure 2 and Table 2).
Table 2 Summary of esophageal stent-related adverse events
| Adverse events | Group 1 (1997-2009) n = 289 (%) | Group 2 (2010-2022) n = 318 (%) | Total n = 607 (%) | p | |
|---|---|---|---|---|---|
| Recurrent dysphagia, in general | 96 (33.2) | 94 (29.6) | 190 (31.3) | 0.337 | |
| Types | Migration | 38 (13.1) | 58 (18.2) | 96 (15.8) | |
| Hyperplasia or cancer | 29 (10.0) | 46 (14.5) | 75 (12.4) | ||
| Food | 6 (2.1) | 8 (2.5) | 14 (2.3) | ||
| Other stent-related causes | 4 (1.4) | 4 (1.3) | 8 (1.3) | ||
| Non-stent-related | 5 (1.7) | 6 (1.9) | 13 (2.1) | ||
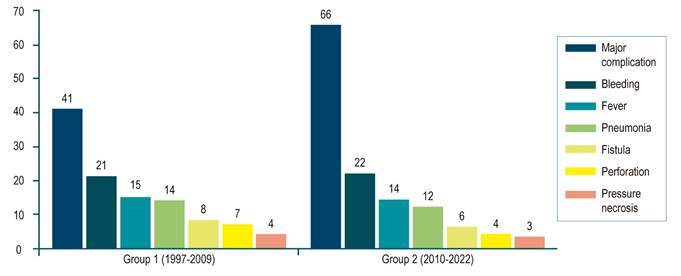
| Patients with major complications | 41 (14.2) | 66 (20.8) | 107 (17.6) | 0.033 | |
| Types | Hemorrhage | 21 (7.3) | 22 (6.9) | 43 (7.1) | |
| Pneumonia | 12 (4.2) | 26 (8.2) | 28 (4.6) | ||
| Perforation | 13 (4.5) | 2 (0.6) | 15 (2.5) | ||
| Fistula | 8 (2.7) | 6 (1.9) | 14 (2.3) | ||
| Pressure necrosis | 4 (1.4) | 3 (1.0) | 7 (1.2) | ||
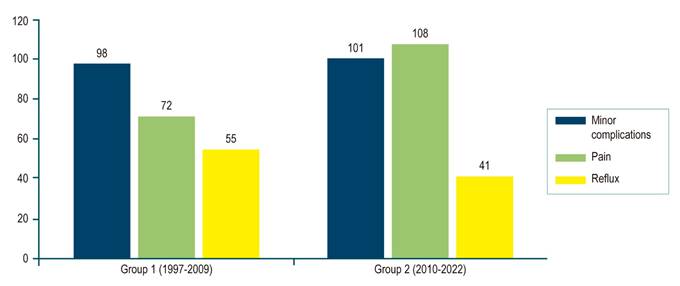
| Patients with minor complications | 98 (33.9) | 101 (31.8) | 199 (32.8) | 0.765 | |
| Types | Pain | 72 (24.9) | 108 (33.9) | 280 (46.1) | |
| Gastroesophageal reflux | 55 (19.0) | 41 (12.9) | 96 (15.8) | ||
| Fever | 15 (5.2) | 14 (4.4) | 29 (4.8) | ||
| Complicated patients, in general | 139 (48.1) | 167 (52.5) | 296 (48.8) | 0.183 | |
Prepared by the authors.
Recurrent dysphagia was diagnosed after a median of 44 days (range: 2-679 days). Univariable Cox regression analysis did not demonstrate a trend toward an increase in recurrent dysphagia over the two periods evaluated (hazard ratio [HR]: 1.01 per 1-year increase; 95% confidence interval [CI]: 0.92-1.05; p = 0.5). Furthermore, no association was found between previous chemotherapy and recurrent dysphagia (HR: 1.22; 95% CI: 0.91-1.81; p = 0.4).
Other adverse events
Almost half of the patients (296; 48.3%) had at least one complication. One hundred seven (17.6%) major complications occurred, including bleeding (n = 43), pneumonia (n = 26), perforation (n = 15), fistula (n = 14), and pressure necrosis (n = 7) (Figure 3 and Table 2). Major complications developed in a median of 11 days after prosthesis insertion (range: 0-557 days). Univariable Cox regression analysis showed no change in major complications over time (HR: 0.99 per 1-year increase; 95% CI: 0.96-1.01; p = 0.21). Trends were noted for major complications with radiotherapy (HR: 1.60; 95% CI: 1.00-2.55; p = 0.05), adenocarcinoma (HR: 0.69; 95% CI: 0.64-1.12; p = 0.07), and younger age (HR: 0.99; 95% CI: 0.97-1.00; p = 0.09).
Pneumonia was the second most common major complication, with 18 of the 28 patients developing pneumonia within four days of stenting, suggesting aspiration during or immediately after the procedure. Pneumonia occurred more frequently in patients who had received prior chemoradiotherapy (18.1%) than in those who had received no initial therapy, chemotherapy alone, or radiation therapy alone (4.2%, 6.3%, and 5.4%, respectively; p = 0.01). The risk was also increased in patients with proximally located stenosis (8.0% vs. 3.2%; p = 0.001).
The risk of developing perforation was significantly higher after pre-prosthesis dilation (13.8% vs. 2.3%; p = 0.04). Dilation was performed to a median of 12 mm. There was a trend toward more frequent bleeding in distal strictures (9.4% vs. 6.0%; p = 0.06). There were 199 (32.8%) minor complications, most related to substernal pain (n = 280, 46.1%) (Figure 4).
The median time to minor complications was two days after stenting (range: 0-267). Cox regression analysis did not reveal an increase in minor complications over time. Other non-significant pain-related associations were younger age, squamous cell histology, previous distal location, and absence of fistula.
Regarding substernal pain, the univariate logistic regression analysis showed a significant increase in the second period (odds ratio [OR]: 1.07; 95% CI: 1.05-1.95; p = 0.014). Therefore, an additional binary multivariable logistic regression analysis was performed, showing that prior chemotherapy or radiotherapy and the absence of a fistula were factors significantly associated with pain. In contrast, adenocarcinoma was independently associated with a lower risk.
Reflux, as a minor complication, showed a significant decrease in its manifestation in the second group evaluated (from 19% to 12.9%, p = 0.048), which is explained by better patient orientation and formulation of acid blockers always on discharge.
Follow-up and survival
At follow-up, 65 patients (10.7%) needed another procedure to achieve enteral nutrition. This included an endoscopic gastrostomy tube in 44 patients (7.2%), a surgically placed gastrostomy tube in 14 patients (2.3%), and a feeding tube placed by interventional radiology in 7 patients (1.2%).
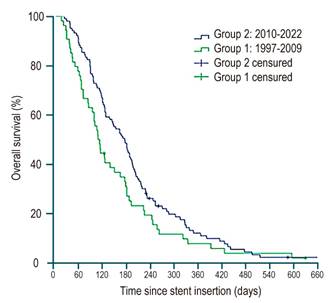
Median overall survival was 169 days (range: 1-1165). At the end of follow-up, 12 patients (2%) were still alive. The majority of patients died as a result of tumor progression (n = 552; 91%), while 14 patients (2.3%) died due to a stent-related complication. No significant differences in survival were detected between the two groups (p = 0.07) (Figure 5).
Discussion
The incidence of esophageal cancer has increased in recent decades. At the same time, endoscopists place an increasing number of esophageal prostheses to palliate malignant obstruction of the esophagus, becoming an effective method to improve dysphagia and the patient’s nutritional status and well-being18. The present study shows a growing increase in the use of esophageal prostheses in various cancer centers over 25 years, providing insight into temporal trends in stenting for malignant esophageal obstruction related to clinical efficacy and safety. A significant technical consideration in the study is the decreased use of fluoroscopy for placement over the years, with good technical and clinical results described in the literature19. However, with advances in endoprosthesis technology (anti-migration, anti-reflux systems, different coverage, diameters, among others), the rate of complications in their placement remains high, and lasting palliation is not achieved in a high proportion of patients20.
The use of stents is highly effective in resolving dysphagia; still, recurrent dysphagia is common and occurs in 31.3% of patients, mainly due to migration or overgrowth of the tumor or hyperplastic tissue. The overall incidence of recurrent dysphagia has not decreased over the years. Indeed, there was a trend toward increasing risk over time, primarily due to a significantly increased risk of stent migration, likely related to the increasing use of fully covered prostheses. A wholly covered stent design substantially increases the risk of migration, which can be explained by less adhesion and fixation to the esophageal wall8. Furthermore, this fully covered design is more favorable to preventing tumor obstruction or excessive hyperplastic growth21. Importantly, our data suggest that the protective effect for tumor or tissue growth outweighs the migration risk because the fully covered design reduces the risk of overall recurrent dysphagia.
Not only recurrent dysphagia but also other adverse events related to prostheses harm patients with already incurable diseases. Complications were observed in almost half of the patients (48.8%), including major complications in 17.6%. We believe that the following findings regarding this topic deserve further attention:
First, we saw a slight decrease in major complications during the second half of the study, with lower rates of bleeding and perforation. However, there was a gradual increase in the proportion of patients treated for more distally located diseases, and the risk of bleeding tends to be higher in these patients than in those with proximal esophageal obstruction.
Second, perforation is another devastating iatrogenic complication. We found that the risk of perforation was significantly higher when dilation had been performed to facilitate stenting. This dilation was most commonly performed in the early days of the series and has now been largely abandoned. Current delivery systems are designed to traverse narrow strictures in a nontraumatic manner, avoiding the need for dilation.
Third, the more significant number of patients treated with chemoradiotherapy before stenting is probably responsible for a greater risk of complications, such as pneumonia in this case, which was more frequent in the second group (4.2% vs. 8.2%, p = 0.04). Interestingly, no significant association could be established for chemotherapy or radiotherapy alone. This suggests a cumulative effect of both treatments. Possible mechanisms for increased susceptibility include pulmonary toxicity from chemoradiotherapy leading to decreased respiratory tract clearance, immunosuppressed state, decreased esophageal motility, and the presence of an esophageal-respiratory fistula22. It is likely that, at least in these cases, procedure-related aspiration has triggered lung infection, emphasizing the importance of close monitoring of the patient during stenting. Nonetheless, considering the lack of alternative palliative measures, we believe that stents can still be recommended to alleviate recurrent malignant dysphagia after chemoradiotherapy, as supported in recent meta-analyses23. The relatively high risk of major complications should be discussed with patients as part of obtaining appropriate informed consent.
Migration is known to be a common and bothersome problem in esophageal stents. Migration rates have not improved over time, even with the introduction of new stents. Currently, anti-migration devices, such as endoscopic sutures, or endoclips, such as Stentfix, are alternatives that are emerging to impact migration rates24. Martins et al. point out that migration occurs in up to 36% of cases of esophageal stenting25.
The migration rate increased in the second period evaluated (from 13.1% to 18.2%, p = 0.113) without being able to be related to a particular type of stent (fully covered, partially covered, or uncovered), as described in other series26.
Retrosternal pain after stent deployment was the most frequently observed minor complication in our cohort in 29.7% of patients. Several explanations can be proposed: First, during the second period evaluated, a prospective study was carried out, which used a symptom diary to evaluate the experience of pain after esophageal stenting27 extended to stents for malignancy. This triggered more accurate and reliable pain recording compared to assessments during previous years. In our practice, we routinely warn patients about the high likelihood of experiencing pain following esophageal stenting.
Second, more patients were pretreated with chemotherapy or radiotherapy, and this was marked as an independent risk factor for retrosternal pain. The exact mechanism to explain pain after pretreatment is unclear. It is conceivable that chemoradiotherapy-induced fibrosis results in lower esophageal wall compliance with relative overstretch and higher pressures after stent expansion compared with patients not treated with chemoradiotherapy.
During follow-up, 72 patients (11.9%) required other enteral nutrition modes. These results demonstrate that while esophageal stenting can be a valuable tool in alleviating dysphagia, it is not always practical, and patients may experience complications at high rates and may ultimately require an alternative to esophageal prostheses.
We must acknowledge several limitations of our study. Our results are based primarily on retrospectively collected data. Therefore, the occurrence of adverse events could have been underestimated. The sample size remains relatively small, which limits the ability to detect any differences that may exist. Additionally, data were missing on some potentially relevant variables; for example, it would have been interesting to evaluate whether the patient’s functional status at follow-up or the stage of the disease has any influence on the clinical outcome and adverse events. Previous studies have denied such a relationship28,29.
Esophageal stenting may allow the introduction of chemoradiotherapy. These therapies allow relief of dysphagia and complete oral nutrition, which is why the European Society of Gastrointestinal Endoscopy recommends their application. However, no clear statement exists on whether they should be introduced before or after stenting30.
Since patients with incurable esophageal cancer have an extremely poor prognosis, the ideal palliative treatment for malignant esophageal strictures should provide rapid and long-lasting relief of symptoms, result in few complications, require a minimal hospital stay, and prolong survival. However, patients who underwent stenting did not often achieve lasting symptom relief due to stent malfunction and had to be readmitted for reoperation. Furthermore, palliation with stents only provides symptom relief but does not prolong survival. Stents with radioactive seed strands have recently been described as combining the advantages of stenting (i.e., faster relief of dysphagia) and brachytherapy (i.e., an advantage in stent patency and survival with a better quality of life)31. Zhu et al.32 proved in a multicenter setting that the placement of esophageal prostheses loaded with I125 radioactive seed strands could produce a modest prolongation of survival in patients with incurable esophageal cancer (177 vs. 147 days; p = 0.005).
Conclusions
The present study demonstrates that esophageal prostheses are an alternative to improve the quality of life of cancer patients with symptoms of dysphagia. Still, they are an imperfect tool, which provides incomplete palliation to many patients, leading to thinking that improving the clinical outcome of stent therapy for malignant esophageal disease is challenging. Although new stent designs have been introduced over time, recurrent dysphagia remains a significant problem, occurring in approximately one-third of patients. Furthermore, changes in management strategies, with more patients pretreated with chemoradiotherapy, are associated with an increase in major complications, primarily pneumonia, but also with the development of substernal pain. Fully covered stents are the most popular option, allowing them to be removed if necessary. In this series, stents did not provide a durable source of enteral access in almost 12% of the cohort. Studies are needed to identify which patients will likely experience stent complications or poor palliation to accommodate technological advances better and enhance patient selection for this still-promising technique.











 texto em
texto em