Introduction
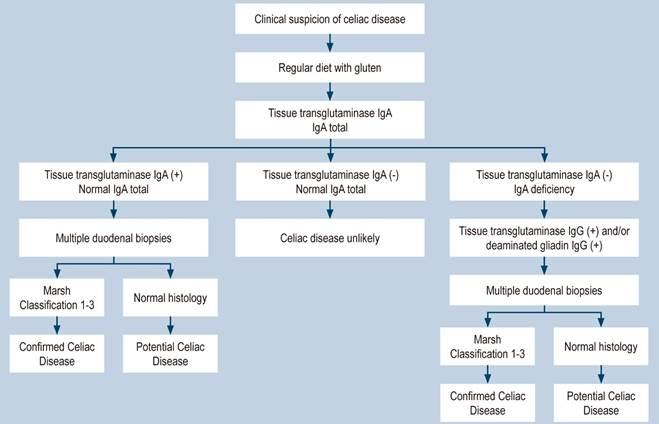
Celiac disease (CD) is a chronic immune-mediated disorder induced by gluten ingestion in genetically predisposed individuals1. Patients may present with classic clinical manifestations (diarrhea, anemia, weight loss) and involvement of other organic systems, such as the neurological, endocrinological, nephrological, and hepatic systems2,3. The diagnostic approach for CD in adults incorporates serological and histological data. Serologic testing for CD should consist of measurement of tissue transglutaminase (tTG) IgA antibodies while following a gluten-containing diet and simultaneous measurement of total IgA since the prevalence of IgA deficiency in patients with CD is 10 to 15 times higher than in healthy subjects4,5. A positive serological test supports the diagnosis, but no test is 100% specific for CD, and diagnostic accuracy varies considerably between laboratories6. The diagnosis of CD is definitively confirmed by lymphocytic infiltrate and villous atrophy in the small intestine biopsy according to the Marsh classification (Figure 1)7.
Active screening for CD is recommended in patients with signs or symptoms suggestive of CD, including diarrhea, weight loss, abdominal pain, bloating, or laboratory abnormalities, such as unexplained elevated serum aminotransferase levels4. It is also recommended in some liver diseases, especially in those with autoimmune disorders, steatosis in the absence of metabolic syndrome, idiopathic non-cirrhotic portal hypertension, cryptogenic cirrhosis, and in the context of liver transplant8,9. Apart from these scenarios, due to the high global prevalence of hepatitis B10, this study aims to review articles in the literature that deal with a potential association between hepatitis B and CD and determine the particularities of the vaccination against hepatitis B in celiac patients.
Materials and methods
The PubMed database was searched in October 2022 to identify articles for this review using two search strategies. In the first strategy (Search A), the descriptor “CD + hepatitis B” was used, and in the second (Search B), “CD + HBV”; only English terms were employed. Additionally, manual searches were carried out in the articles’ references. We included articles on CD and hepatitis B with a clearly described methodology, published in English-language journals without date restrictions, and selected by affinity with the objective. A full-text version of selected articles was obtained to confirm eligibility.
Hepatitis B and CD
Some authors have investigated the prevalence of CD in patients with hepatitis B; these findings are summarized in Table 111-14. The prevalence of CD varied between 3.3% and 17.2%6-8. Leonardi et al.11 demonstrated a high prevalence of CD in patients with hepatitis B. Although limited by the small size of the patients studied, this study is interesting because it may represent what has been observed in Italy15. The prevalence of hepatitis B virus (HBV) in Italy is higher than in the rest of Europe16, and a high prevalence of CD is also estimated17. Concerning the study by Nau et al., southwest Brazil is also a region with a high prevalence of hepatitis B, where the majority of inhabitants are descendants of Portuguese, Italian, and German immigrants18.
Despite the limited sample size of their hepatitis B cohorts, which requires conservative interpretation, the studies still provide intriguing data. Soto Iglesias et al.19 presented two patients who developed CD after resolving an acute HBV infection. Reactive serological tests and the presence of typical histopathological findings confirmed the diagnosis of CD. The same authors suggested that developing the immune response for clearance of HBV triggers the intestinal tissue damage observed in CD in genetically predisposed individuals. One hypothesis in the field of liver disorders is that a deregulated immune process would induce liver damage due to autoantibodies. Another hypothesis suggests that liver damage results from increased intestinal permeability, resulting in toxins or autoantigens from the liver through the portal vein12. Although the role of HBV infection in the development of autoimmune diseases has been widely discussed in the literature, it remains a controversial topic.
Bardela et al. found the opposite: The prevalence of HBV among 158 individuals with CD was 4.5%20. Other studies that evaluated the prevalence of HBV in celiac patients are summarized in Table 1 21-23. After all these years, there is no clinical evidence of an association between CD and hepatitis infection, and the appearance of these two diseases in a patient may be a chance finding. Still, despite the findings described in this review, it is not possible to make a specific recommendation for CD screening in people with hepatitis B or vice versa24.
Table 1 Prevalence of celiac disease in patients with hepatitis B and vice versa
| Author | Year | Country | Patients | Total | N | Prevalence |
|---|---|---|---|---|---|---|
| CD in patients with hepatitis B | ||||||
| Leonardi et al.11 | 2010 | Italy | Patients with hepatitis B | 35 | 6 | 17.2% |
| Sima et al.12 | 2010 | Iran | Patients with hepatitis B | 88 | 8 | 9.1% |
| Nau et al.13 | 2013 | Brazil | Patients with hepatitis B | 50 | 6 | 12% |
| Sood et al.14 | 2017 | India | Patients with hepatitis B | 30 | 1 | 3.3% |
| Hepatitis B in patients with CD | ||||||
| Bardella et al.20 | 1995 | Italy | Patients with CD and elevated aminotransferases | 67 | 3 | 4.5% |
| Novacek et al.21 | 1999 | Austria | Patients with CD | 178 | 1 | 0.6% |
| Moghaddam et al.22 | 2013 | United Kingdom | Patients with CD | 98 | 1 | 1% |
| Tanwar et al.23 | 2020 | India | Patients with CD and portal hypertension | 42 | 2 | 4.8% |
Interferon α
Interferon α and its pegylated form have been used for more than thirty years to treat chronic hepatitis B with the advantages of a finite duration of treatment and a loss of hepatitis B surface antigen (HBsAg) with antibody seroconversion against the sustained hepatitis B virus surface antigen (anti-HBsAg); however, efficacy is limited since seroconversion is achieved in a small proportion of treated patients, and side effects are frequent24. Leonardi et al.11 evaluated 15 of 60 patients who had used interferon α therapy for 12 months at a dose of 5 million units (5 MU). As mentioned above, it demonstrated a high prevalence of hepatitis B among celiac patients but did not compare the prevalence of hepatitis B according to interferon α use. Sima et al.12 investigated 88 patients with chronic hepatitis B. They observed that 26 patients who had previously used interferon α had celiac antibodies compared to 6 patients without treatment with interferon α (p < 0.05). Some reports indicate autoimmune disorders such as insulin-dependent diabetes mellitus and CD that may develop during treatment with interferon α for viral hepatitis because this drug has immunomodulatory properties that can induce a silent autoimmune disorder such as CD25-29.
Interferon α therapy may trigger CD in susceptible patients, and it has been hypothesized that the most likely pathogenesis of this process could be a dysregulation of the balance between the need to recognize antigens of pathogenic microorganisms and the need to prevent inappropriate immune responses to foods and normal flora12. These findings suggest that CD should be sought before interferon therapy for early diagnosis and prevention of CD complications. However, there is still insufficient evidence that interferon α can activate CD.
HBV vaccine
The success of a vaccination program depends on the availability of safe and highly effective vaccines and the implementation of appropriate vaccination strategies. After a complete vaccination cycle with the classic schedule of three vaccine doses administered at 0, 1, and 6 months, anti-HBsAg seroprotection rates at a concentration equal to or greater than 10 mIU/mL (the antibody threshold considered protective) are close to 100% in healthy children and almost 95% in healthy adults30,31.
Along with host-related factors (i.e., age, sex, immunocompetence, genetics, and co-infections), vaccine and vaccination-related factors have also been found to affect the response to vaccination. Among these, the dose and vaccination schedule, the injection site, and the route of administration are critical factors in achieving an optimal immune response32.
Addressing the issue of CD and hepatitis B is mainly related to immunization against hepatitis B in people with CD. The CD is more common in individuals with HLA-DQ2 and HLA-DQ8, and the literature has shown that these individuals have a lower response rate to HBV vaccination than the general population33-36. In particular, the immune response to the HBV vaccine is primarily determined by immunogenetic peptides through the HLA-DR and DQ molecules, and the DR3-DQ2 and DR7-DQ2 haplotypes generally have a lower response rate37-39.
The correlation between CD activity (by measuring serum anti-transglutaminase titers) and the development of an antibody response to the HBV vaccine has been previously demonstrated34. Trovato et al.40 evaluated 96 children with CD; 41.7% (n = 40) showed non-protective or absent antibody titers against HBV. Elevated tTG-IgA values (p = 0.023) and older age at diagnosis (p < 0.001) were associated with a lack of seroconversion to the HBV vaccine. They hypothesize that competition between gluten and the HBV surface antigen could explain this phenomenon. Therefore, we can speculate that in patients with CD, the immune system may focus on the non-self antigen that occurs most frequently in these patients (dietary gluten) and may polarize its activity in this direction rather than toward the antigen HBV surface antigen with massive production of tTG autoantibodies, but suboptimal production of antibodies against HBV surface antigens.
A lack of response has also been correlated with age, smoking, obesity, and male sex39. However, when children with CD follow a gluten-free diet, the immune response to the HBV vaccine is similar to that of the general population. This suggests that treatment adherence may improve the lack of response to the HBV vaccine in celiac children41. Lastly, the lack of response to the hepatitis B vaccine should be considered a sign of possible undiagnosed CD33. Nemes et al.34 evaluated 128 children and adolescents with CD and 113 age-matched controls: 22 patients with CD were prospectively immunized after diagnosis during dietary treatment (Group 1) and a total of 106 celiac children, and the control subjects received vaccination by mass immunization regardless of diet status (Group 2). Diet compliance and CD activity were monitored by measuring tTG and anti-endomysial antibodies (EmA). The vaccine response rate for Group 1 was 95.5% compared to 50.9% for Group 2. The response rate among 27 undiagnosed and untreated CD patients was 25.9%, significantly lower than that in control subjects of 75.2% (p < 0.001).
Some strategies can be followed when vaccinating against hepatitis B in CD patients. One possibility would be administering the three usual doses, giving booster doses to non-responders, and performing serology to assess the response after each dose42. The intradermal route for the booster dose of the hepatitis B vaccine in celiac patients is a better option to obtain a higher titer of antibodies against HBV43. Furthermore, the intradermal route allows for a better cost-effectiveness ratio since the cost reduction exceeds 50% (2 μg per dose) compared to standard intramuscular vaccination (10 μg per dose)44. A third strategy would be to revaccinate celiac patients intramuscularly in treatment with a gluten-free diet after the decrease in celiac-specific antibodies34. The intradermal route is preferable for revaccination of these patients45.
The prevalence of seroprotective levels of anti-HBsAg detected 11 years after primary immunization and the frequency of response to a booster dose of the vaccine are lower in celiac patients than in healthy controls46. Therefore, a booster dose of the vaccine should be administered every ten years to all celiac patients to protect non-responding celiacs from HBV infection45.
Conclusion
Despite the coexistence of both diseases, a clear association between hepatitis B and CD has not been demonstrated, so routine screening for CD in HBV carriers cannot be recommended; however, hepatitis B should be investigated in the setting of elevated aminotransferases in celiac patients. Due to the poor response to vaccination against HBV, particular strategies should be implemented in celiac patients, such as the intradermal route and revaccination.











 text in
text in