Introduction
Obesity has been a public health problem for decades, which has caused the development of multiple treatments, including malabsorptive surgery, with increasing frequency since 1966 due to better results in terms of weight loss and metabolic control. However, changes secondary to bariatric procedures increase the frequency of calculous cholecystitis by up to 36%, with 15% of stones lodged in the bile duct. Endoscopic retrograde cholangiopancreatography (ERCP) is the procedure of choice for managing calculous pathology of the bile duct and for instrumentation of biliopancreatic obstructive diseases, including malignant disease; however, endoscopic access to the duodenal papilla in the altered anatomy of gastric bypass results in an enormous technical challenge, given the sui generis anatomical alteration that implies a long afferent loop.
In our setting, this very frequently translates into surgical management, either for bile duct exploration or laparoscopic transgastric ERCP.
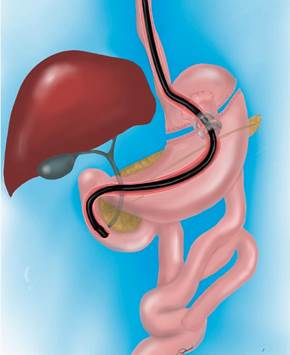
This report describes a case of ERCP in a patient with gastric bypass, in which communication was created under endosonographic guidance between the gastric reservoir and the stomach left in the peritoneal cavity, allowing access to the papilla with a usual side-viewing instrument. The procedure was performed in the advanced endoscopy unit of Estudios Endoscopios SAS in Medellín without transferring the patient to the surgical unit with close outpatient follow-up. This directly impacts hospitalization times, patient comfort, and associated costs.
Clinical case
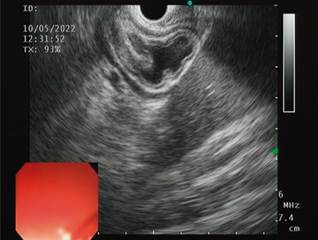
We present the case of a 32-year-old female patient with a history of gastric bypass surgery for morbid obesity. After the associated weight loss, the patient had an episode of abdominal pain with an ultrasound diagnosis of cholelithiasis, which required a laparoscopic cholecystectomy one year before the current consultation. On admission, the patient reported abdominal pain in the upper quadrants, predominantly on the right, associated with mild jaundice and choluria without acholia. With suspected residual choledocholithiasis, the patient was taken for magnetic resonance cholangiography, which failed to identify the causes of pain associated with jaundice. A biliopancreatic endoscopic ultrasound (EUS) was requested, which showed a bile duct of standard diameter with an oval echogenic image of 3.8 mm in diameter, floating and with acoustic shadowing upon the alpha maneuver from the first scanning station (the other stations were not evaluable due to the history of bypass; Figure 1). This finding is compatible with choledocholithiasis, for which ERCP is indicated.
Source: Patient’s medical record.
Figure 1 Initial diagnostic EUS. The cursor points to a small stone in the alpha maneuver from the first evaluation station.
The possibility of ERCP by laparoscopic transgastric enteroscopy and EUS-directed transgastric ERCP (EDGE) is analyzed (Figure 2).
Given the background in the literature and our experience, which reveals a high risk of failed enteroscopy procedures associated with the length of the alimentary loop, we decided to rule out this option.
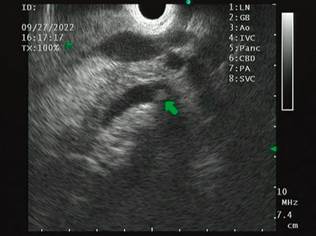
The possibility of a laparoscopic approach is discussed, which is not imperative since the patient has had a cholecystectomy. It is also considered that the patient does not want new surgical interventions or their inherent risks. We then opted for EUS-directed management since, during the diagnostic EUS, the accessibility of the remnant stomach was verified in the proximity of the gastric reservoir and the afferent loop (Figure 3).
At the time, the patient had no symptoms, so we decided to perform the procedure in two stages: in the first stage, an EUS-guided jejunogastrostomy with apposing stenting, which allowed the complete expansion of the stent and maturation of the tract. In the second stage, one week after stenting, consisting of the usual ERCP, the side-viewing duodenoscope was advanced.
The first stage was performed under sedation, with EUS and fluoroscopic guidance. The remnant stomach was traced near the distal jejunum 10 mm from the gastrojejunal anastomosis. EUS-guided puncture with a 19G needle, instillation of water-soluble contrast medium in distilled water, and a gastrogram were performed. A 0.035-inch hydrophilic guidewire was advanced through the puncture needle, deploying the 15-mm apposing stent with a hot-tip introducer (Hot Axios 15 mm, Boston) in four steps. The deployment of the proximal tab in the afferent loop required further advancement of the pusher for release, given the limited space in this segment, which prevents the tip from getting away from the intestinal wall. The location of the stent was verified endoscopically with a slim front-viewing gastroscope (Figure 4).
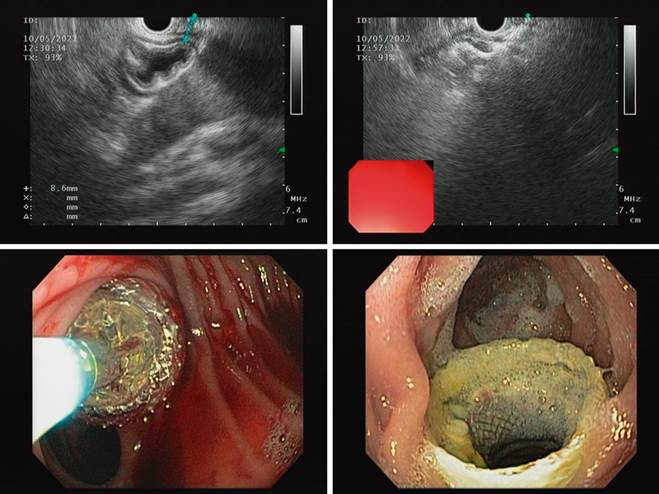
Source: Patient’s medical record.
Figure 4 Step-by-step frames of the procedure until the mature jejunogastrostomy after one week.
In-hospital follow-up was carried out for 24 hours, with the absence of symptoms and adequate tolerance to the oral route, so the patient was discharged with an outpatient appointment for ERCP.
The second stage was performed ten days later under sedation and after gastroscopy to verify adequate passage to the duodenum. The side-viewing duodenoscope was advanced through the apposing stent to the duodenal papilla, and the usual ERCP was performed with a sufficient papillotomy and stone extraction. There was no bleeding or complication during the procedure (Figure 5). Upon removal of the duodenoscope, the position of the apposing stent was verified, and emptying of the gastric chamber was performed by aspiration.
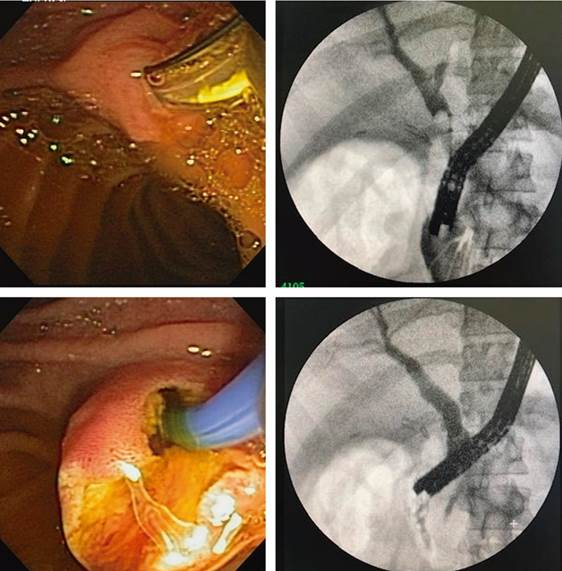
Source: Patient’s medical record.
Figure 5 Access to the papilla through the apposing stent and cholangiogram with a side-viewing duodenoscope. Standard technique.
The patient was managed on an outpatient basis through telephone follow-up; she did not exhibit alarm symptoms or abdominal pain and had adequate tolerance to the oral route.
The stent was left in position for two more weeks; it was removed endoscopically without difficulty, and the jejunogastric fistula was confirmed to be contracting.
Discussion
In the second half of the 20th century and the first two decades of this century, the prevalence of obesity has resulted in a growing number of treatments to try to control its frequency and lethal consequences.
Medical treatments, including diet and lifestyle changes, have proven to have limited effectiveness, so surgical therapies have been used with increasing frequency and variable success rates but are more satisfactory in the medium term. In our setting, bariatric surgical management has opted for gastric sleeve surgery and Roux-en-Y gastric bypass surgery (RYGB).
In the United States, RYGB accounts for 70-80% of bariatric procedures, and it has been estimated that between 29% and 36% of these patients will develop gallstone disease associated with rapid weight loss, primarily 12 to 18 months postoperative1,2.
For more than 40 years, ERCP has been the standard for managing choledocholithiasis; however, post-surgical anatomical distortion in patients with RYGB significantly limits the endoscopic approach3.
ERCP in patients with altered anatomy poses an enormous technical challenge and is associated with a significant number of failed procedures and adverse events when compared to the standard procedure. The most used approaches today can be classified as totally endoscopic, balloon or double-balloon enteroscopy-assisted (BEA-ERCP), EUS-assisted (EDGE), and mixed, endoscopic and laparoscopic techniques (laparoscopic-assisted transgastric ERCP)4,5.
The endoscopic approach to the bile duct in these cases has several difficulties. The first is the endoscopic approach to the papilla. RYGB involves the creation of a bile loop of 80 to 150 cm, meaning maneuvering an endoscopic instrument requires very high skill and training. Even so, angulations of the loop generated by peritoneal adhesions or internal hernias can make it impossible to reach the duodenum in a retrograde manner.
Once the papilla is reached, the second difficulty level corresponds to selective bile duct cannulation, which must be performed inverted and with a front-viewing instrument. In addition, the usual accessories cannot be used on the enteroscopy because they lack the necessary length or diameter.
Lastly, successful permeabilization is difficult once the bile duct has been canalized. The lack of instruments specifically designed to deal with difficult stones, including the impossibility of implanting plastic or metal prostheses, can make the entire procedure fruitless6.
The technical success of the enteroscopy approach in patients with RYGB is disappointing in statistical analyses, as it fluctuates between 69% and 74%; clinical success is even lower, between 60% and 65%7.
Izawa et al., in a retrospective study with 91 patients with different types of gastroenteric reconstruction, report a success rate of 92.3% for the papilla approach, 90.5% for cannulation, but only 78% success for the complete procedure8.
Baron and Vickers described the creation of a gastrostomy to access the gastric remnant through which double-balloon enteroscopy-assisted ERCP (DBE-ERCP) was performed. This technique is more effective in accessing the pancreaticobiliary tree, but it has difficulties regarding the maturation time of the gastrostomy and a high rate of complications associated with it9.
Laparoscopic-assisted transgastric ERCP is a proven technique, initially described in 200210, which approaches the papilla in an antegrade manner with a side-viewing duodenoscope and standard endoscopic instruments; this increases the success of the procedure. It is necessary to access the excluded stomach by laparoscopy and create a gastrostomy through which the side-viewing duodenoscope is advanced, which must be guided by the laparoscopist for passage through the pylorus; subsequently, a technique similar to that performed in procedures with preserved anatomy must be followed.
A 2012 single-center retrospective study by Schreiner et al. found that laparoscopic-assisted ERCP was more effective than enteroscopy-assisted ERCP in patients with long-loop RYGB11.
In 2014, Baron et al. performed percutaneous endoscopic gastrostomies and placed self-expanding metal stents to allow immediate transgastric ERCP12.
Recently, with the emergence of apposing metal stents, communication of abdominal cavities has been achieved through EUS and fluoroscopy, allowing successful management of pancreatic necrosis and performance of gastroenteric bypasses in patients with obstruction of the gastric tract outlet. Performing EUS-guided gastrogastrostomy or jejunogastrostomy to access the remnant stomach in patients with RYGB is a technique proposed in 2014 in a series of cases published by Kedia et al.13; since then, the refinement of the method has allowed it to be performed more and more frequently and has multicenter experiences that support its feasibility and adoption.
In our country, no reports in the literature allow for evaluating the technique’s performance, which is why the description of this clinical case is valuable.
A systematic review from 2022, conducted by Prakash et al. (14) with 169 patients, reported that the technique was successful in creating the gastrogastric or jejunogastric fistula in 99% of cases, and ERCP was completed in 98%.
Minor adverse events associated with stent migration or malposition during the procedure and mild abdominal pain afterward are described in 18% of cases. Moderate adverse events occurred in 5%, including bleeding, persistent fistula, and perforation. Only one serious event is listed, which was a gastric perforation that required surgery.
A common question is secondary weight gain. An American multicenter study of 13 groups on the East Coast collected data from 178 patients taken for EDGE and mainly evaluated the persistence of fistula. It was objective in only nine cases, five of which were successfully managed with endoscopic closure. Three patients exhibited weight gain during the study, and this method found a success rate of 98% for the resolution of biliary pathology.
If these results are compared with those published in 2017 by Banerjee et al. regarding laparoscopic-assisted transgastric ERCP with a success rate in access to the papilla of 100% and ductal cannulation of 98.5%15, it is not possible to sense apparent differences with the EDGE; however, comparative studies are required.
EDGE offers the possibility of using an utterly endoscopic technique under sedation on an outpatient basis, so, despite the high cost of apposing stents, it may find validity in future cost analyses.
Conclusion
EUS-directed ERCP is a procedure with an adequate safety profile in patients with biliary obstruction and a history of gastric bypass; it can be performed on an outpatient basis and in the comfort of the endoscopy room. There are limitations regarding the available studies; most have been published as case reports and systematic reviews. None describes the experience in our environment, so this initial report is valuable as an index case for implementing the method.











 texto em
texto em