Introduction
Celiac disease (CD) is a chronic enteropathy of the small intestine mediated by exposure to gluten in the diet. Worldwide, an annual increase of 7.5% has been reported due to greater recognition of the disease, screening methods, and improvement in diagnostic techniques1. Its manifestation is varied with both gastrointestinal and extraintestinal signs and symptoms, and the non-classical form is the most common in more than half of the cases, which makes its exploration a challenge2. There is an association between CD and autoimmune diseases, the most common of which are type I diabetes mellitus, thyroiditis, and autoimmune hepatitis3. It has been noted that patients with systemic lupus erythematosus (SLE) have a prevalence four times higher than the general population and share a predisposition to certain specific genotypes (DQ2, DQ8, B8, and DR3) and environmental factors4,5.
As stated, CD has various forms of clinical manifestation and can occur in coexistence with systemic autoimmune rheumatic diseases. In this case, we describe the condition of a patient with SLE in remission, in whom the study of chronic diarrhea resulted in the diagnosis of CD, obtaining an excellent response to removing gluten from her diet.
Case presentation
We present the case of a 22-year-old female patient from an urban area, a psychology student with a history of SLE diagnosis at the age of 10, who exhibited cutaneous, hematological, immunological, and neuropsychiatric signs; she was in clinical remission under outpatient rheumatology follow-up and was being managed with cyclosporine, rituximab, and oral methylprednisolone. Previously, she showed intolerance to hydroxychloroquine and adverse events with azathioprine. Besides, seven years ago, a case of diarrhea was attributed to the use of mycophenolate mofetil; a colonoscopy plus a biopsy was performed at that time, which reported ileitis and colitis with intraepithelial lymphocytosis. Although the medication was discontinued at the time, she continued to have diarrheal stools with malabsorption characteristics, which is why she was diagnosed with irritable bowel syndrome with a predominance of diarrhea and no response to conventional management.
Furthermore, vitamin B12 deficiency and iron deficiency anemia have been documented in recent years. The patient consulted the emergency department due to a two-month history of generalized paresthesias, muscle spasms, myalgias, and episodes of tetany in the previous week. Upon admission to the emergency department, postprandial diarrhea persisted, with 5 to 6 stools a day. At her initial evaluation, she was in poor general condition (hemodynamically stable, hydrated, anxious, with mucocutaneous paleness, generalized rigidity, positive Trousseau, and Chvostek sign). She was transferred to the intensive care unit (ICU) for monitoring and management.
The laboratory tests, upon admission, showed severe hypocalcemia: ionic calcium of 0.6 mmol/L (normal range (NR), 1.15-1.33 mmol/L), serum calcium of 5.4 mg/dL (NR, 8,6-10 mg/dL), albumin of 4 g/dL (NR, 3.5-5.2 g/dL), normal parathyroid hormone: 25 pg/mL (NR, 10-65 pg/mL), vitamin D deficiency: 18.8 ng/mL (NR, >30 ng/mL), severe hypomagnesemia: 0.59 mEq/L (NR, 1.59-2.56 mg/dL), and mild hypokalemia: 3.1 mmol/L (NR, 3.5-5.5 mmol/L). So, intravenous replacement of calcium, magnesium, and potassium was initiated.
Four months earlier, an upper GI endoscopy had been performed, which was reported as normal with negative Helicobacter pylori. The possibility that chronic diarrhea was a manifestation of SLE activity was considered, so a magnetic resonance enterography was performed to evaluate possible involvement, extension, and location; still, it was reported within normality. The laboratory tests did not show the consumption of serum complement (C3, C4), the anti-double-stranded DNA antibodies were negative, and no active urinary sediment was found. There were no findings other than the anemia in the blood count that suggested SLE activity given by calculators such as SLEDAI-2K and a usually high rate of complications such as vasculitis or lupus enteritis, which is why these latter diagnoses and the activity of the underlying disease were ruled out.
Exposure to toxins and infectious causes such as viral, bacterial, and parasitic, as well as the human immunodeficiency virus (HIV) and infection by Clostridium difficile and Giardia lamblia, among others, were ruled out. The differential diagnosis between CD and other causes of malabsorption, such as chronic pancreatitis and Crohn’s disease, was then considered. Due to the suspected diagnosis, the following serological studies were requested: anti-tissue transglutaminase IgA antibodies (0.2 U/mL, NR <10 U/mL), antigliadin IgA (<0.1 U/mL, NR <20 U/mL) and IgG (<0.5 U/mL, NR <20 U/mL), with normal values and low levels of total IgA (0.02 g/Lm, NR: 0.7-4 g/L). Enteroscopy did not find pancreatic alterations but showed erosive bulboduodenitis, with villous atrophy and an increase in intraepithelial lymphocytes in the bulboduodenal and distal biopsy which, together with the immunohistochemical study, allowed us to conclude the diagnosis of Marsh 3a type CD (Figures 1 and 2).
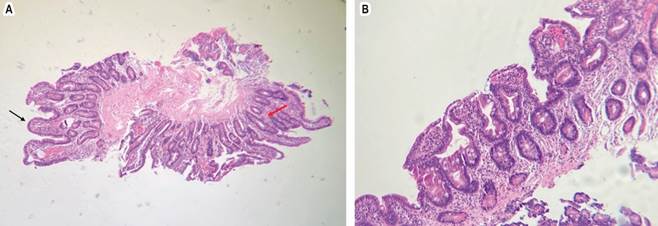
Source: authors’ archive.
Figure 1 H-E x100. A. Moderate widening and flattening of some villi (black arrow). In other areas, it has preserved villi but with hyperplasia of the crypts (red arrow). B. Moderate villous atrophy.
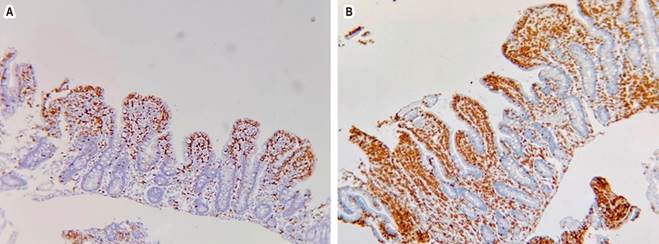
Source: Authors’ archive.
Figure 2 Immunohistochemical study with CD3 (A) and CD8 (B). An increase in intraepithelial lymphocytes with more than 40 x 100 cells.
This was reaffirmed with the positive clinical response to management with a gluten-free diet (GFD), and 15 days after discharge, control of gastrointestinal and muscular symptoms and correction of nutritional deficiencies was achieved. SLE, for its part, remained in continuous remission.
Discussion
Patients with SLE may have gastrointestinal manifestations; nausea and vomiting are more common (53%), followed by anorexia (49%) and abdominal pain (19%)6. However, within the differential diagnosis of abdominal pain and diarrhea with malabsorption clinical characteristics, exocrine pancreatic insufficiency, bacterial overgrowth in the small intestine, liver disease, and CD should be considered6; the latter shares an autoimmune nature with SLE, but its coexistence remains rare7.
In CD, intestinal malabsorption can manifest with weight loss, hypoproteinemia, early osteoporosis, fat-soluble vitamins and minerals, iron deficiency, and vitamin B12 deficiency, as in our patient’s case8. Severe hypocalcemia and tetany may be found in around 10%9. The mechanism of hypocalcemia in CD is complex and multifactorial; it depends on the loss of villous surface resulting from villous atrophy, malabsorption of fatty acids, deterioration of intestinal calcium transport mechanisms due to calbindin deficiency in the enterocytes, deterioration in calcium absorption due to vitamin D deficiency, and hypomagnesemia secondary to malabsorption, with the consequent alteration of the parathyroid hormone both in its production and in its function, which further exacerbates the condition of hypocalcemia10.
In diagnosing CD, serological tests should always be performed with gluten ingestion. They should include serum levels of total IgA, given that the deficiency of this immunoglobulin can give false negative results. If this occurs, anti-deamidated gliadin peptide IgG or anti-transglutaminase IgG antibodies could be helpful; however, they are usually negative3. Endoscopic findings in this context take on particular importance. Esophagogastroduodenoscopy can reveal changes in the mucosa: villous atrophy, areas of cracked mucosa, and mosaic pattern, among others, which can be so subtle as to go unnoticed. For this reason, its exploration is recommended even if the appearance of the mucosa is normal2.
Despite being rare, seronegative CD is the most common cause of seronegative villous atrophy (SNVA), representing 31% to 45% in antibody-negative patient cohorts2,11,12. In this subtype of patients, it is necessary to consider other less frequent causes such as infections in up to 27%, usually Giardia, but also Helicobacter pylori, HIV, tuberculosis; autoimmune disorders such as Hashimoto’s thyroiditis, Crohn’s disease, Sjögren’s syndrome, primary biliary cholangitis; enteropathy due to drugs, especially non-steroidal anti-inflammatory drugs (NSAIDs) and angiotensin II inhibitors. There are particularities in this population; seronegative CD occurs in women later in life, around the age of 50, in white ethnic groups (in non-white races, up to two-thirds of villous atrophies are of infectious cause)11,12. Furthermore, in the immunohistochemistry of all patients with SNVA, there is positive staining for cytotoxic intraepithelial lymphocytes T-CD8; however, positive staining for T-CD4 is documented in patients with a non-CD cause of SNVA that can mimic refractory CD11.
The SNVA presented here occurred in a woman much younger than what was reported in the seronegative CD and of mixed ethnicity; however, she had the typical pattern on immunohistochemistry, with positive staining for T-CD8 and negative staining for T-CD4. In the SNVA, the histopathological examination of the duodenal biopsy with multiple sampling currently emerges as the most conclusive test for CD and, together with the response to GFD, provides, as in the case of the patient, a reliable diagnosis2. Aziz et al. demonstrated that, in seronegative CD, survival is lower compared to seropositive CD in a 14-year follow-up, malabsorption is expected, and they are more related to other coexisting autoimmune diseases. Therefore, they require a more extensive study and closer follow-up11. In this specific group of patients, reports of severe hypocalcemia are not known, and it is a particularity of the case described.
In patients with SLE, it has been found that antigliadin antibodies are the most frequent, sensitive, and specific13,14. However, it must be considered that, as reported by Zidouni et al.15, not all cases of SLE and CD are seropositive; conversely, Rensch et al.13 studied 103 patients with SLE: 24 were positive for antigliadin antibodies, but no endoscopic or histological evidence of CD was found, which suggests that in SLE there is a hyperexpression of antigliadin antibodies, they are false positives in 23% and are not associated with CD. Considering that studies have estimated a prevalence of CD of 3% in patients with SLE, five times higher than in the general population, regardless of seropositivity, it is essential to use endoscopic and histological findings, HLA-DQ2/DQ8, and the response to gluten withdrawal to make a reliable diagnosis of CD. SLE can manifest before or after CD, with an average margin of five years, with diarrhea, abdominal pain, nausea/vomiting, recurrent oral aphthosis, and anemia, which should be taken as signs and symptoms to suspect this association14.
Conclusion
Cases of seronegative CD are not typical and require a complete study of other entities that can generate SNVA in the non-white population, mainly infectious but also toxic and immune. Its lower survival, more significant association with other autoimmune diseases, and malabsorption make close follow-up imperative to avoid complications as severe as the reported hypocalcemia. CD should be suspected in patients with SLE who present with diarrhea, abdominal pain, nausea/vomiting, recurrent oral aphthosis, and anemia. Endoscopic and histological studies must be used, and the response to gluten suspension must be evaluated, given the probability of false positives and negatives with antibodies such as antigliadin. Due to the excellent prognosis with treatment, early diagnosis can improve quality of life and reduce morbidity.











 texto em
texto em 



