Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Agronomía Colombiana
Print version ISSN 0120-9965
Agron. colomb. vol.28 no.1 Bogotá Jan./Apr. 2010
FISIOLOGIA DE CULTIVOS
Hermann Restrepo-Díaz1, 4, Juan Carlos Melgar2, and Leonardo Lombardini3
1 Departamento de Agronomía, Facultad de Agronomía, Universidad Nacional de Colombia, Bogotá (Colombia).
2 Citrus Research and Education Center, University of Florida, Lake Alfred, FL (United States).
3 Department of Horticulture, Texas A&M University, College Station, TX (United States).
4 Corresponding author. hrestrepod@unal.edu.co
Received for publication: 17 December, 2009. Accepted for publication: 5 March, 2010
ABSTRACT
Horticultural crops include a wide range of commodities, such as fruits and vegetables, that are highly valuable for humanity. They are extensively grown worldwide, and their production can be described as an open and highly complex system affected by many factors, among which we can count weather, soil and cropping system, as well as the interaction between these factors. The aim of environmental physiology is to characterize the interaction between environmental stress and crop response, in order to maximize both yield quantity and quality. This review presents the most recent findings about the effects of the main abiotic environmental factors (light, temperature, and water) on whole plant physiology of horticultural crops. Environmental stresses can cause morpho-anatomical, physiological and biochemical changes in crops, resulting in a strong profit reduction. A clear understanding of environmental factors and their interaction with physiological processes is extremely important for improving horticultural practices (irrigation, light management, mineral nutrition, greenhouse design, etc.), optimizing photosynthetic carbon assimilation and increasing fruit productivity and crop quality. In addition, the information obtained by ecophysiological studies can be incorporated into breeding programs or agricultural zoning strategies.
Key wods: vegetable crops, fruit trees, water stress, temperature.
RESUMEN
Los productos hortícolas como frutas y vegetales son ampliamente cultivados, dado que incluyen un extenso abanico de alimentos de gran valor para la humanidad. Los sistemas hortícolas son abiertos y altamente complejos, y se ven afectados por factores como el clima, el suelo y el sistema de producción, así como por la interacción entre estos factores. Por lo anterior, la importancia de la fisiología ambiental o ecofisiología radica en que permite caracterizar la interacción entre los factores de estrés ambiental y la respuesta de los cultivos, con el propósito de obtener una producción exitosa. El objetivo de esta revisión consiste en reunir los resultados de las investigaciones más recientes acerca del efecto de los factores ambientales abióticos (luz, agua y temperatura) sobre la respuesta fisiológica de los cultivos hortícolas. Los factores de estrés ambiental pueden causar distintos cambios morfológicos, fisiológicos y bioquímicos en los cultivos, determinando una considerable reducción en su rendimiento. La comprensión de la interacción entre estos factores ambientales y procesos fisiológicos es importante en el mejoramiento de las prácticas hortícolas (riego, manejo de la luz, nutrición mineral, diseño de infraestructuras, etc.), con el objetivo de optimizar la fotosíntesis e incrementar la productividad de los cultivos. Adicionalmente, la información que se obtiene mediante la ecofisiología es una herramienta útil en los programas de mejoramiento genético, o en estrategias de ordenación del territorio agrícola.
Palabras clave: hortalizas, árboles frutales, estrés térmico, sequía.
Introduction
Widely cultivated for the high value of their products, horticultural crops include fruits and vegetables which provide essential food, minerals and vitamins that are critical to human nutrition (Kwack, 2007). The production of horticultural crops can be characterized as an open and highly complex system affected by climate, soil, cropping system and interactions between these factors (Lentz, 1998). Given that plant growth and development are directly and indirectly influenced by environmental factors (Schaffer and Andersen, 1994), in order to obtain a successful production it is essential to understand clearly how said factors affect plant physiology (Wien, 1997). In this context, ecophysiology is the science that studies the interactions between plants and their physical, chemical and biotic environment (Larcher, 2003; Lambers et al., 2008). Environmental physiology is also important to study both the effect of different environmental stresses (shading, heavy metals, drought and salinity, among others) on growth and development (Salisbury and Ross, 1994) and the way plants compensate the detrimental effects of stress through different mechanisms (stress response, acclimation and adaptation) (Taiz and Zeiger, 2006).
Environmental physiology studies have been extensively used to improve the management of certain species or to explain differences among cultivars (Higgins et al., 1992; Hampson et al., 1996; Campostrini and Glenn, 2007; Sagaram et al., 2007; Lombardini et al., 2009). Nevertheless, in regions where agriculture is not very modern, or where new horticultural crops are introduced, the information supplied by environmental physiology studies is highly valuable for deciding on the distribution and performance of crops (Higgins et al., 1992). Knowledge on the responses of horticultural crops to environmental factors such as temperature, water availability, light or carbon dioxide (CO2) concentration is useful to determine the effect of suboptimal environmental conditions and to manage crops for maximum productivity (Schaffer and Andersen, 1994). In addition, a better understanding of the interaction between environmental factors and physiological processes contributes to horticultural breeding programs, production sustainability improvement and efficient agricultural zoning (Campostrini and Glenn, 2007).
Thus, the aim of this review is to gather the most recent information on the effects of environmental factors (light, temperature and water) on whole plant physiology of horticultural crops as expressed by growth, yield, fruit quality and photosynthetic features.
Light
Sunlight is not only the energy source for photosynthesis, but also the most important factor affecting productivity in horticultural crops (Papadopoulos and Pararajasingham, 1997; Gregoriu et al., 2007). Carbon exchange rate (CER) is strongly dependent on irradiance, absorption, and utilization of photon energy (Jackson, 1980; Gregoriu et al., 2007). Low irradiance, in as much as it determines insufficient light penetration into the canopy, influences CER directly by reducing photon energy utilization, thus decreasing productivity (Hampson et al., 1996; Gregoriu et al., 2007). Canopy management as a routine activity in horticultural crops is aimed at increasing light interception and productivity, stabilizing yield, and improving fruit quality (Hampson et al., 1996).
Given that they need sunlight for flowering and fruit bud formation, fruit-tree crops keep a balance between light interception and light distribution (Huett, 2004). Since the relationship between photosynthetic photon flux density (PPFD) and net photosynthesis provides basic information for modeling leaf, plant, or canopy growth (Hanson et al., 1987), several studies have focused on light interception and distribution into the canopy (Higgins et al., 1992; Wood, 1996; Huett, 2004; Lombardini, 2006a). Light interception modeling has also been important in the development of pruning and training techniques for optimizing yield, and of tree removal strategies aimed at improving orchard productivity (Garriz et al., 1998; Huett, 2004; Li and Lakso, 2004; Lombardini et al., 2006a). A summary of the photosynthetic performance of several fruit-tree crops is listed in Tab. 1.
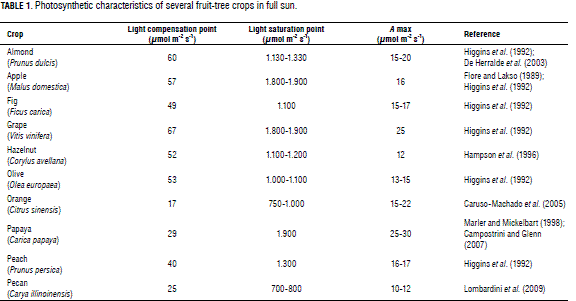
Shading (levels of 60% to 90%) affects leaf morphology and anatomy, gas exchange and water relations (water use efficiency, stomatal conductance, and thus photosynthesis) in horticultural crops (Bjorkman, 1981; Atanasova et al., 2003; Heuvel et al., 2004; Gregoriu et al., 2007). In addition, shade diminishes reproductive potential directly by decreasing flowering, fruit set and fruit size; and indirectly by reducing the vegetative growth that the plant needs to support reproduction (Hampson et al., 1996). A summary of the effects of shading on several horticultural crops is reported in Tab. 2.

Previous studies have shown the importance of plant response to shading, since this information is useful to determine ideal plant density, cropping systems or growth conditions in greenhouses (Papadopoulos and Pararajasingham, 1997; Francescangeli et al., 2006; Francescangeli et al., 2007; Callejón-Ferre et al., 2009). Francescangeli et al. (2007) observed that shading increased growth cycle duration and diminished net assimilation rate in broccoli. However, as individual plant relative growth rate (RGR) was almost constant, they concluded that broccoli can be considered as a shade-tolerant plant, thus apt for intercropping systems. Tsubo and Walker (2004) and Nasrullahzadeh et al. (2007) studied the effect of intercropped beans and observed that dry mass was 40% lower in shaded plants (shading was up to 90%). Nevertheless, shading did not have significant effects on yield parameters (number of pods and number of grains per plant, and number of grains per pod). These authors concluded that growing beans in agroforestry or intercropping systems would be advantageous for farmers. Regarding planting distance, close spacing has been observed to have a negative effect on fruit set in tomato, apparently due to an inadequate supply of photosynthates (Papadopoulos and Pararajasingham, 1997). In a 4-year study conducted in tomato by Zahara and Timm (1973), the variables stem diameter, fruit set, number of flowers and number of leaves per plant decreased as plant density was increased up to 96.3 plants/m2. Similar results were found by Papadopoulos and Ormrod (1990), who observed that tomato fruit set declined with decreased plant spacing (i.e. 58%, 52% and 13% fruit set at 60 cm, 45 cm and 23 cm spacing, respectively).
In horticultural production systems, plants can experience water loss due to high solar radiation levels, often causing irreversible burns (Castilla, 2005). Shading is a useful strategy for reducing leaf temperature, fruit damage or water loss at irradiance peaks; and for growing shade-tolerant species in areas with excessive radiation (Kittas et al., 1999).
In a 2-year study, Callejón-Ferre et al. (2009) evaluated the effects of using aluminized screens with different degrees of shading (40, 50, and 60%) as well as traditional whitewashing conditions on the production and quality of tomato cv. Atletico grown under greenhouse conditions. The results showed that 60% shading improved fruit firmness but decreased the amount of soluble solids.
Temperature
Temperature is an important factor influencing seed germination, vegetative growth, flowering, fruit set and fruit ripening in horticultural crops (Sage and Kubien, 2007; Ledesma et al., 2008; Kositsup et al., 2009). Both high and low temperatures, be they temporary or constant, can induce morpho-anatomical, physiological and biochemical changes in plants, leading to profit reduction (Higuchi et al., 1998; Wang et al., 2003; Wahid et al., 2007). Heat stress can be a concern in many regions of the tropics and subtropics, since high temperature can cause significant damage such as sunburns on leaves, branches and stems, anticipated leaf senescence and abscission, shoot and root growth inhibition and fruit discoloration and damage (Yamada et al., 1996a; Higuchi et al., 1998; Almeida and Valle, 2007; Wahid et al., 2007). Reproductive processes are also highly affected by heat stress in most plants (Wahid et al., 2007). Through observations in strawberry, Ledesma et al. (2008) found that high temperature stress negatively affected the number of inflorescences, flowers and fruits, and that plant response to high temperature stress was cultivar dependent. In tomato, pollen germination and pollen tube growth, ovule viability, stigma and style positions and number of pollen grains retained by the stigma were also seriously affected by high temperature (Foolad, 2005). In cherimoya, warm temperatures determined the production of low-viability pollen; and therefore of asymmetrical and small fruits containing few seeds (Higuchi et al., 1998). However, it has been observed that pollen viability is reduced in papaya when the temperature drops below 20°C. This condition can also cause problems of sex change and low-sugar content in fruits (Galán-Saúco and Rodríguez-Pastor, 2007). In cacao, temperatures above 23°C seem to accelerate vegetative flushing initiation (Almeida and Valle, 2007). Regarding anatomical changes, symptoms observed under heat stress conditions are generally similar to those checked under water stress. Plants present reduced cell size, closure of stomata, curtailed water loss, increased stomatal and trichome densities and greater xylem vessels in both root and shoot (Añón et al., 2004; Wahid et al., 2007). In rose, significant increases in stomatal index and in stomatal and epidermal cell density were observed in plants grown under high temperature (Pandey et al., 2007).
Studies conducted by Wentworth et al. (2006) in common beans showed high temperature dependent increases in leaf thickness, palisade development and stomatal density in the adaxial surface of the leaves. In a work conducted by Zhang et al. (2005) in grapes, they found that warm temperatures considerably affected the mesophyll cells, increased plasma membrane permeability, enhanced the loss of grana stacking and determined the swelling of stroma lamellae. Furthermore, an increase in the concentration of abscisic acid (ABA) was observed in grape leaves due to high temperature, suggesting that ABA may be a high-temperature acclimation and heat-tolerance induction factor in this crop (Abass and Rajashekar, 1993). As previously mentioned, heat stress evidently affects the anatomical structures from tissue to sub-cellular levels. Thus, the accumulation of all these changes under high temperature stress may result in poor plant growth and productivity.
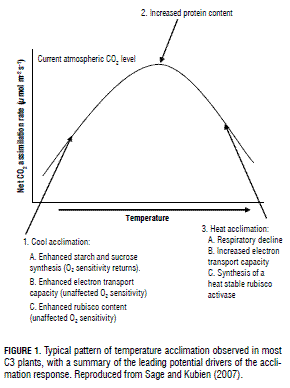
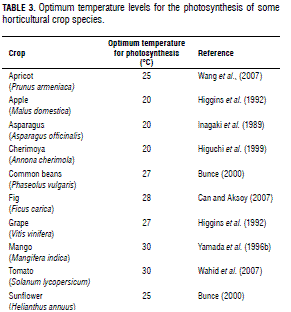
High temperature induces the acclimation of photosynthesis by changing the photosynthetic capacity, the temperature response of photosynthesis or both (Fig. 1) (Sage and Kubien, 2007; Wang et al., 2007; Kositsup et al., 2009). Changes in several photosynthetic characteristics under high temperatures are excellent indicators of plant tolerance to heat stress (Wahid et al., 2007), which is indeed capable of damaging the thylakoid membranes (Petkova et al., 2007). As a consequence, a series of physiological parameters such as chlorophyll fluorescence, variable to maximum fluorescence ratio (Fv/Fm) and base fluorescence (F0) can be used to estimate heat tolerance in different species or cultivars (Yamada et al., 1996a). Studies realized by Petkova et al. (2007) indicated that chlorophyll fluorescence induction parameters (F0, Fm, Fv and their ratios) are good indicators of heat tolerance in common beans, and can therefore be used to trace characters of interest in breeding programs. Similar results have been reported by Nyarko et al. (2008) in cabbage. Changes in Fv/Fm ratio under heat stress conditions could also be a good indicator in screening heat-resistant grape cultivars (Kadir et al., 2007). High temperatures influence photosynthetic capacity and stomatal conductance by decreasing the activation state of rubisco. Furthermore, heat stress diminishes the amount of photosynthetic pigments (Wahid et al., 2007). In tomato, the latter condition (temperature above 45°C for 2 h) injured the plasma membrane, altered the pigment composition of the photosynthetic apparatus, and caused an important reduction of the net photosynthetic rate due to affections in the Calvin cycle and the functioning of photosystem II (Camejo et al., 2005). In citrus species, net CO2 assimilation rate is reduced by partial decrease in both stomatal conductance and instantaneous carboxylation efficiency at temperatures above or below the optimum range (28-32°C) (Machado et al., 2005). Hence, knowledge about temperature levels is useful in physiological research as well as horticultural crop production. In general, optimum temperature levels have been obtained for many horticultural crops through laboratory and/or field experiments. Understanding the way this factor affects plant physiology is greatly desirable to avoid damages due to unfavorable temperatures during plant ontogeny (Wahid et al., 2007). A summary of optimum temperature levels for the photosynthesis of several horticultural crop species is shown in Tab. 3.
Water
Since water is fundamental for maintaining normal physiological activity and membrane transport processes (Jones and Tardieu, 1998), supplying it adequately is crucial for obtaining maximum productivity of horticultural crops. In addition, water plays an important role in horticultural crops, since fruits and vegetables are usually sold on a fresh weight basis and yield is predominantly determined by water content (Marcelis et al., 1998). Drought stress occurs when there is not enough soil water content for successful growth or water supply replenishment (Larcher, 2003; Lombardini, 2006b). A decline in leaf relative water content (RWC) initially causes stomatal closure, which in turn leads to a decrease in the supply of CO2 to the mesophyll cells and thus reduces leaf photosynthetic rate. Likewise, drought stress also affects processes such as cell division and expansion, ABA synthesis and sugar accumulation, consequently reducing crop yield (Marsal and Girona, 1997; Chartzoulakis et al., 1999; Raviv and Blom, 2001; Arquero et al., 2006; Lombardini, 2006b).
In general, it can be said that horticultural crops require a high water supply through appropriate irrigation schedules. Nevertheless, deficit irrigation can enhance fruit quality by raising dry matter percentage and sugar content (Jones and Tardieu, 1998; Spreer et al., 2007). Furthermore, controlled water deficit has been used as a technique to stimulate blossoming in crops such as guava or litchi, or to substitute for adequate chilling when temperate crops such as apple are grown in the tropics (Chaikiattiyos et al., 1994). Hence, regulated deficit irrigation (RDI) and partial rootzone drying (PRD) techniques have been applied to withhold water during certain periods, thus producing moderate drought stress, which in turn has improved yield, fruit quality and water use efficiency. The results of RDI experiments have been contradictory, but sometimes promising (Lombardini et al., 2004; Spreer et al., 2007). In experiments conducted in Spain, RDI has increased grape productivity (Faci et al., 2009) and citrus fruit quality (Ballester et al., 2009), although the yield effect has been controverted for some species (Robles et al., 2009). RDI can also be used to delay flowering and harvesting time (Melgar et al., 2008) or to increase flowering and productivity at certain periods of the year when prices are high. Such is the case of the "forzatura", a traditional practice applied in lemon crops in Sicily, where the summer bloom is accentuated by withholding irrigation until the trees wilt (Barbera et al., 1985). It is necessary, however, to determine the optimum stress level so that the dry period does not have depressing effects on tree vitality, and to understand the interactions among tree water status, crop load and fruit growth, in order to optimize yield under water deficit conditions. For example, high yields can be obtained in peach with deficit irrigation if an appropriate management of fruit thinning is done at stage III of fruit growth. This is so because said management enhances fruit size not only due to a reduction in fruit competition, but to an improvement in tree water status as well (Marsal et al., 2006; López et al., 2006, 2007).
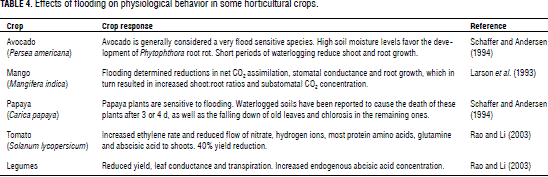
On the other hand, it is important to discuss about flooding, since plant development is affected by either too little or too much water in the root zone. Flooding is produced by storms, over irrigation, poor drainage, high water tables and dam and river overflowing (Rao and Li, 2003). As it has been previously mentioned, plants induce a series of physical, chemical and biological processes in response to stress conditions. Under flooding conditions, plants show similar symptoms to those they develop under heat or water stress. Plant responses to waterlogging include increased internal ethylene concentration, low stomatal conductance, decrease in leaf, root and shoot development, changes in osmotic potential and nutrient uptake, and reduced chlorophyll content and photosynthesis (Tamura et al., 1996; Ashraf and Rehman, 1999; Rao and Li, 2003; Issarakraisila et al., 2007). Flooding also increases the severity of certain diseases, mainly root-rotting fungi (Rao and Li, 2003), as reported by De Siva et al. (1999) regarding Phytophthora root rot in blueberry. The decrease of oxygen level in soils affects the bioavailability of nutrients as well as the ability of root systems to uptake and transport water and mineral nutrients (Lizaso et al., 2001). Waterlogging caused inhibition of N uptake from the soil and reduced leaf concentrations of N, P, K, Ca and Mg in avocado (Schaffer and Andersen, 1994) and pea (Rao and Li, 2003). The effects of flooding duration on some horticultural crops are summarized in Tab. 4.
Conclusion
It can be said that knowledge about the interactions between environmental factors and plant physiology facilitates the identification of environmental changes such as lack of light, high temperatures or water deficit. For example, the shading of horticultural crops can reduce photosynthesis rate, transpiration and stomatal density and conductance; and enhance flower abortion. Likewise, high temperatures can affect pollen viability and germination, number of flowers and number of fruits per plant. Finally, ecophysiological information is a tool that can be used in breeding programs to obtain improved cultivars, as well as in strategies of agricultural zoning, thus enhancing productivity.
Literature cited
Abass, M. and C.B. Rajashekar. 1993. Abscisic-acid accumulation in leaves and cultured cells during heat acclimation in grapes. HortScience 28, 50-52. [ Links ]
Almeida, A.A.F. and R.R. Valle. 2007. Ecophysiology of cacao tree. Braz. J. Plant Physiol. 19(4), 425-448. [ Links ]
Aloni, B., L. Karni, Z. Zaidman, and A.A. Schaffer. 1996. Changes of carbohydrates in pepper (Capsicum annuum L.) flowers in relation to their abscission under different shading regimes. Ann. Bot. 78, 163-168. [ Links ]
Arquero, O., D. Barranco, and M. Benlloch. 2006. Potassium starvation increases stomatal conductance in olive trees. HortScience 41(2), 433-436. [ Links ]
Ashraf, M. and H. Rehman. 1999. Mineral nutrient status of corn in relation to nitrate and long-term waterlogging. J. Plant Nutr. 22(8), 1253-1268. [ Links ]
Atanasova, L., D. Stefanov, I. Yordanov, K. Kornova, and L. Kavardzikov. 2003. Comparative characteristics of growth and photosynthesis of sun and shade leaves from normal and pendulum walnut (Juglans regia L.) trees. Photosynthetica 41, 289-292. [ Links ]
Ballester, C., J. Castel, and J.R. Castel. 2009. Riego deficitario controlando en Clementina de nules y Navel lane late: producción y calidad de la fruta. Acta Hort. 54, 198-202. [ Links ]
Bañón, S., J.A. Fernández, J.A. Franco, A. Torrecillas, J.J. Alarcón, and M.J. Sánchez-Blanco. 2004. Effects of water stress and night temperature preconditioning on water relations and morphological and anatomical changes of Lotus creticus plants. Scientia Hort. 101, 333-342. [ Links ]
Barbera, G., G. Fatta del Bosco, and B. Lo Cascio. 1985. Effects of water stress on lemon summer bloom: the forzatura technique in the Sicilian citrus industry. Acta Hort. 171, 391-397. [ Links ]
Bjorkman, O. 1981. Responses to different quantum flux densities. pp. 57-107. In: Lange, O.L., P.S. Nobel, C.B. Osmond, and H. Ziegler (eds.). Encyclopedia of plant physiology (New Series). Springer-Verlag, Berlin. [ Links ]
Bunce, J.A. 2000. Acclimation of photosynthesis to temperature in eight cool and warm climate herbaceous C3 species: Temperature dependence of parameters of a biochemical photosynthesis model. Photosynthesis Res. 63(1), 59-67. [ Links ]
Callejón-Ferre, A.J., F. Manzano-Agugliaro, M. Díaz-Pérez, A. Carreño-Ortega, and J. Pérez-Alonso. 2009. Effect of shading with aluminised screens on fruit production and quality in tomato (Solanum lycopersicum L.) under greenhouse conditions. Spanish J. Agr. Res. 7, 41-49. [ Links ]
Camejo, D., P. Rodríguez, A. Morales, J.M. DellAmico, A. Torrecillas, and J.J. Alarcón. 2005. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant Physiol. 162(3), 281-289. [ Links ]
Campostrini, E. and D. Glenn. 2007. Ecophysiology of papaya: A review. Braz. J. Plant Physiol. 19(4), 413-424. [ Links ]
Can, H.Z. and U. Aksoy. 2007. Seasonal and diurnal photosynthetic behaviour of fig (Ficus carica L.) under semi-arid climatic conditions. Acta Agr. Scand. Section B - Soil Plant Sci. 57, 297-306. [ Links ]
Caruso-Machado, E., P.T. Schmidt, C. Lázaro-Medina, and R. Vasconcelos-Ribeiro. 2005. Respostas da fotossíntese de três espécies de citros a fatores ambientais. Pesq. Agropec. Bras. 40(12), 1161-1170. [ Links ]
Castilla, N. 2005. Invernaderos de plástico. Tecnología y manejo. Ediciones Mundi-prensa, Madrid. [ Links ]
Chaikiattiyos, S., C.M. Menzel, and T.S. Rasmussen. 1994. Floral induction in tropical fruit trees: effects of temperature and water supply. J. Hort. Sci. 69, 397-415. [ Links ]
Chartzoulakis, K., A. Patakas, and A.M. Bosabalidis. 1999. Changes in water relations, photosynthesis and leaf anatomy induced by intermittent drought in two olive cultivars. Environ. Exp. Bot. 42(2), 113-120. [ Links ]
De Herralde, F., C. Biel, and R. Savé. 2003. Leaf photosynthesis in eight tree almond cultivars. Biol. Plant 46(4), 557-561. [ Links ]
De Pinheiro, A.R., and L.F.M. Marcelis. 2000. Regulation of growth at steady-state nitrogen nutrition in lettuce (Lactuca sativa L.): Interactive effects of nitrogen and irradiance. Ann. Bot. 86, 1073-1080. [ Links ]
De Siva, A., K. Patterson, C. Rothrock, and R. McNew. 1999. Pythophthora root rot of blueberry increases with frequency of flooding. HortScience 34, 693-695. [ Links ]
Faci, J., O. Blanco, and J. Negueroles. 2009. Efecto del riego deficitario controlado aplicado desde el envero a recolección en la producción y calidad de uva de mesa Autumn royal. Acta Hort. 54, 192-197. [ Links ]
Flore, J.A. and A.N. Lakso. 1989. Environmental and physiological regulation of photosynthesis in fruit crops. Hortic. Rev. 11, 111-157. [ Links ]
Foolad, M.R. 2005. Breeding for abiotic stress tolerances in tomato. pp. 613-684. In: Ashraf, M. and P.J.C. Harris (eds.). Abiotic stresses: plant resistance through breeding and molecular approaches. The Haworth Press Inc., New York, NY. [ Links ]
Francescangeli, N., M.A. Sangiacomo, and H. Martí. 2006. Effects of plant density in broccoli on yield and radiation use efficiency. Scientia Hort. 119, 135-143. [ Links ]
Francescangeli, N., M.A. Sangiacomo, and H. Martí. 2007. Vegetative and reproductive plasticity of broccoli at three levels of incident photosynthetically active radiation. Spanish J. Agric. Res. 5(3), 389-401. [ Links ]
Galán-Saúco, V.G. and M.C.R. Rodríguez-Pastor. 2007. Greenhouse cultivation of papaya. Acta Hort. 740, 191-195. [ Links ]
Garriz, P.I., G.M. Colavita, and H.L. Álvarez. 1998. Fruit and spur leaf growth and quality as influenced by low irradiance levels in pear. Scientia Hort. 77, 195-205. [ Links ]
Gregoriu, K., K. Pontikis, and S. Vemmos. 2007. Effects of reduced irradiance on leaf morphology, photosynthetic capacity and fruit yield in olive (Olea europaea L.). Photosynthetica 45(2), 172-181. [ Links ]
Hampson, C.R., A.N. Azarenko, and J.R. Potter. 1996. Photosynthetic rate, flowering and yield component alteration in hazelnut in response to different light environments. J. Amer. Soc. Hort. Sci. 121, 1103-1111. [ Links ]
Hanson, P.J., R.E. McRoberts, J.G. Isebrands, and R.K. Dixon. 1987. An optimal sampling strategy for determining CO2 exchange rate as a function of photosynthetic flux density. Photosynthetica 21, 98-101. [ Links ]
Heuvel, J.E.V., J.T.A. Proctor, K.H. Fisher, and J.A. Sullivan. 2004. Shading affects morphology, dry-matter partitioning and photosynthetic response of greenhouse-grown 'Chardonnay' grapevines. HortScience 39, 65-70. [ Links ]
Higuchi, H., N. Utsunomiya, and T. Sakuratani. 1998. High temperature effects on cherimoya fruit set, growth and development under greenhouse conditions. Scientia Hort. 77, 23-31. [ Links ]
Higuchi, H., T. Sakuratani, and N. Utsunomiya. 1999. Photosynthesis, leaf morphology, and shoot growth as affected by temperatures in cherimoya (Annona cherimola Mill.) trees. Scientia Hort. 80, 91-104. [ Links ]
Higgins, S.S., F.E. Larsen, R.B. Bendel, G.K. Radamaker, J.H. Bassman, W.R. Bidlake, and A.A. Wir. 1992. Comparative gas exchange characteristics of potted, glasshouse-grown almond, apple, fig, grape, olive, peach and Asian pear. Scientia Hort. 52, 313-329. [ Links ]
Huett, D.O. 2004. Macadamia physiology review: a canopy light response study and literature review. Aust. J. Agric. Res. 55, 609-624. [ Links ]
Inagaki, N., K. Tsuda, and S. Maekawa. 1989. Effects of light intensity, CO2 concentration, and temperature on photosynthesis of Asparagus officinalis L. J. Japan. Soc. Hort. Sci. 58(2), 369-376. [ Links ]
Issarakraisila, M., Q. Ma, and D.W. Turner. 2007. Photosynthetic and growth responses of juvenile Chinese kale (Brassica oleracea var. alboglabra) and Caisin (Brassica rapa subsp. parachinensis) to waterlogging and water deficit. Scientia Hort. 111, 107-113. [ Links ]
Jackson, J.E. 1980. Light interception and utilization by orchard systems. Hort. Rev. 2, 208-267. [ Links ]
Jones, H.G. and F. Tardieu. 1998. Modelling water relations of horticultural crops: a review. Scientia Hort. 74, 21-46. [ Links ]
Kadir, S., M. von Weihe, and K. Al-Khatib. 2007. Photochemical efficiency and recovery of photosystem II in grapes after exposure to sudden and gradual heat stress. J. Amer. Soc. Hort. Sci. 132(6), 764-769. [ Links ]
Kittas, C., A. Baille, and P. Giaglaras. 1999. Influence of covering material and shading on the spectral distribution of light in greenhouses. J. Agr. Eng. Res. 73, 341-351. [ Links ]
Kositsup, B., P. Montpied, P. Kasemsap, P. Thaler, T. Ameglio, and E. Dreyer. 2009. Photosynthetic capacity and temperature responses of photosynthesis of rubber trees (Hevea brasiliensis Müll. Arg.) acclimate to changes in ambient temperatures. Tree Physiol. 23, 357-365. [ Links ]
Kwack, B.H. 2007. The value of human life with horticultural practices and products. Acta Hort. 762, 17-21. [ Links ]
Lambers, H., F.S. Chapin and T.L. Pons. 2008. Plant physiological ecology. 2nd ed. Springer, Berlin. [ Links ]
Larcher, W. 2003. Physiological plant ecology. 4th ed. Springer, Berlin. [ Links ]
Larson, K.D., B. Schaffer, and F.S. Davies. 1993. Physiological, morphological and growth responses of mango trees to flooding. Acta Hort. 342, 152-159. [ Links ]
Ledesma, N.A., M. Nakata, and N. Sugiyama. 2008. Effect of high temperature stress on the reproductive growth of strawberry cvs. Nyoho and Toyonoka. Scientia Hort. 116, 186-193. [ Links ]
Lentz, W. 1998. Model applications in horticulture: a review. Scientia Hort. 74, 151-174. [ Links ]
Li, K.T. and A.N. Lakso. 2004. Phosynthetic characteristics of apple spur leaves after summer pruning to improve exposure to light. HortScience 39, 969-972. [ Links ]
Lizaso, J.I., L.M. Meléndez, and R. Ramírez. 2001. Early flooding of two cultivars of tropical maize. II. Nutritional responses. J. Plant Nutr. 24(7), 997-1011. [ Links ]
Lombardini, L. 2006a. One-time pruning of pecan trees induced limited and short-term benefits in canopy light penetration, yield, and nut quality. HortScience 41, 1469-1473. [ Links ]
Lombardini, L. 2006b. Chapter 4: Ecophysiology of plants in dry environments. pp. 47-66. In: P. DOdorico and A. Porporato (eds.). Dryland ecohydrology. Springer, Berlin. [ Links ]
Lombardini, L., H.W. Caspari, D.C. Elfving, T.D. Auvil, and J.R. McFerson. 2004. Gas exchange and water relations in Fuji apple trees grown under deficit irrigation. Acta Hort. 636, 43-50. [ Links ]
Lombardini, L., H. Restrepo-Díaz, and A. Volder. 2009. Photosynthetic light response and epidermal characteristics of sun and shade pecan leaves. J. Amer. Soc. Hort. Sci. 134(3), 372-378. [ Links ]
López, G., M. Mata, A. Arbones, J.R. Solans, J. Girona, and J. Marsal. 2006. Mitigation of effects of extreme drought during stage III of peach fruit development by summer pruning and fruit thinning. Tree Physiol. 26, 469-477. [ Links ]
López, G., J. Girona, and J. Marsal. 2007. Response of winter root starch concentration to severe water stress and fruit load and its subsequent effects on early peach fruit development. Tree Physiol. 26, 469-477. [ Links ]
Machado, E.C., P.T. Schmidt, C.L. Medina, and R.V. Ribeiro. 2005. Photosynthetic responses of three citrus species to environmental factors. Pesq. Agropec. Bras. 40(12), 1161-1170. [ Links ]
Marcelis, L.F.M., E. Heuvenlink, and J. Goudriaan. 1998. Modeling biomass production and yield of horticultural crops: a review. Scientia Hort. 74, 83-111. [ Links ]
Marler, T.E. and M.V. Mickelbart. 1998. Drought, leaf gas exchange and chlorophyll fluorescence of field-grown papaya. J. Amer. Soc. Hort. Sci. 123(4), 714-718. [ Links ]
Marsal, J. and J. Girona. 1997. Effects of water stress cycles on turgor maintenance processes in pear leaves (Pyrus communis). Tree Physiol. 17, 327-333. [ Links ]
Marsal, J., G. López, M. Mata, and J. Girona. 2006. Branch removal and defruiting for the amelioration of water stress effects on fruit growth during stage III of peach fruit development. Scientia Hort. 108, 55-60. [ Links ]
Martínez-Vega, R.R., G. Fischer, A. Herrera, B. Chaves, and O.C. Quintero. 2008. Características físico-químicas de frutos de feijoa influenciadas por la posición en el canopi. Rev. Colomb. Cienc. Hortic. 2(1), 21-32. [ Links ]
Melgar, J.C., J. Dunlop, and J.P. Syvertsen. 2008. Winter time drought stress delays Valencia flowering and avoids young fruit loss during late season mechanical harvesting. p. 1208. In: Proc. ASHS Annual Conference. Orlando, FL. [ Links ]
Nasrullahzadeh, S., K. Ghassemi-Golezani, A. Javanshir, M. Valizade, and M.R. Shakiba. 2007. Effects of shade stress on ground cover and grain yield of faba bean (Vicia faba L.). J. Food Agric. Environ. 5(1), 337-340. [ Links ]
Nishizawa, T., A. Ito, Y. Motomura, M. Ito, and M. Togashi. 2000. Changes in fruit quality as influenced by shading of netted melon plants (Cucumis melo L. Andesu and Luster). J. Japan. Soc. Hort. Sci. 69(5), 563-569. [ Links ]
Nyarko, G, P.G. Alderson, J. Craigon, E. Murchie, and D.L. Sparkes. 2008. Comparison of cell membrane thermostability and chlorophyll fluorescence parameters for the determination of heat tolerance in ten cabbage lines. J. Hort. Sci. Biotech. 83(5), 678-682. [ Links ]
Pandey, R., P.M. Chacko, M.L. Choudhary, K.V. Prasad, and M. Pal. 2007. Higher than optimum temperature under CO2 enrichment influences stomata anatomical characters in rose (Rosa hybrida). Scientia Hort. 113, 74-81. [ Links ]
Papadopoulos, A.P. and D.P. Ormrod. 1990. Plant spacing effects on yield of the greenhouse tomato. Can. J. Plant Sci. 70, 565-573. [ Links ]
Papadopoulos, A.P. and S. Pararajasingham. 1997. The influence of plant spacing on light interception and use in greenhouse tomato (Lycopersicon esculentum Mill.): A review. Scientia Hort. 69, 1-29. [ Links ]
Petkova, V., I.D. Denev, D. Cholakov, and I. Porjazov. 2007. Field screening for heat tolerant common bean cultivars (Phaseolus vulgaris L.) by measuring of chlorophyll fluorescence induction parameters. Scientia Hort. 111, 101-106. [ Links ]
Rahman, H.U., P. Hadley, S. Pearson, and M.D. Dennett. 2007. Effect of incident radiation integral on cauliflower growth and development after curd initiation. Plant Growth Regul. 51, 41-52. [ Links ]
Rao, R. and Y.C. Li. 2003. Management of flooding effects on growth of vegetable and selected field crops. HortTecnology 13(4), 610-616. [ Links ]
Raviv, M. and T.J. Blom. 2001. The effect of water availability and quality on photosynthesis and productivity of soil-less grown cut roses. Scientia Hort. 88(4), 257-276. [ Links ]
Robles, J.M., J.G. Pérez-Pérez, I. García-Oller, E. Arques, J.M. Berna, and P. Botía. 2009. Respuesta del limonero Fino 49 al riego deficitario. Efectos sobre el crecimiento, la producción y la calidad del fruto. Acta Hort. 54, 236-240. [ Links ]
Sagaram, M., L. Lombardini, and L.J. Grauke. 2007. Variation in leaf anatomy of pecan cultivars from three ecogeographic locations. J. Amer. Soc. Hort. Sci. 132, 592-596. [ Links ]
Salisbury, F.B. and C.W. Ross. 1994. Fisiología vegetal. Grupo Editorial Iberoamericana, México, DF. [ Links ]
Sage, R. and D. Kubien. 2007. The temperature response of C3 and C4 photosynthesis. Plant Cell Environ. 30, 1086-1106. [ Links ]
Schaffer, B. and P.C. Andersen. 1994. Handbook of environmental physiology of fruit crops. Vol. II: Sub-tropical and tropical crops. CRC Press. Boca Raton, FL. [ Links ]
Spreer, W., M. Nagle, S. Neidhart, R. Carle, S. Ongprasertand, and J. Muller. 2007. Effect of regulated deficit irrigation and partial root zone drying on the quality of mango fruits (Mangifera indica L., cv. Chok Anan). Agr. Water Manage. 88, 173-180. [ Links ]
Taiz, L. and E. Zeiger. 2006. Plant physiology. 4th ed. Sinauer Associates, Sunderland, MA. [ Links ]
Tamura, F., K. Tanabe, M. Katayama, and A. Itai. 1996. Effects of flooding on ethanol and ethylene production by pear rootstocks. J. Japan Soc. Hort. Sci. 65(2), 261-266. [ Links ]
Thiagarajan, A., R. Lada, and P. Joy. 2007. Compensatory effects of elevated CO2 concentration on the inhibitory effects of high temperature and irradiance on photosynthetic gas exchange in carrots. Photosynthetica 45(3), 355-362. [ Links ]
Tsubo, M. and S. Walker. 2004. Shade Effects on Phaseolus vulgaris L. intercropped with Zea mays L. under well-watered conditions. J. Agron. Crop Sci. 190, 168-176. [ Links ]
Wahid, A., S. Gelani, M. Ashraf, and M.R. Foolad. 2007. Heat tolerance in plants: an overview. Environ. Exp. Bot. 61, 199-223. [ Links ]
Wang, W., B. Vinocur, and A. Altman. 2003. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218, 1-14. [ Links ]
Wang, F.L., H. Wang and G. Wang. 2007. Photosynthetic responses of apricot (Prunus armeniaca L.) to photosynthetic photon flux density, leaf temperature, and CO2 concentration. Photosynthetica 45(1), 59-64. [ Links ]
Wentworth, M., E.H. Murchie, J.E. Gray, D. Villegas, C. Pastenes, M. Pinto, and P. Horton. 2006. Differential adaptation of two varieties of common bean to abiotic stress. II. Acclimation of photosynthesis. J. Exp. Bot. 57(3), 699-709. [ Links ]
Wien, H.C. 1997. The physiology of vegetable crops. CAB International, Wallingford, UK. [ Links ]
Wood, B.W. 1996. Canopy morphology of pecan cultivars. HortScience 31, 139-142. [ Links ]
Yamada, M., T. Hidaka, and H. Fukamachi. 1996a. Heat tolerance in leaves of tropical fruit crops as measured by chlorophyll fluorescence. Scientia Hort. 67, 39-48. [ Links ]
Yamada, M., H. Fukumachi, and H. Tetsushi. 1996 b. Photosynthesis in longan and mango as influenced by high temperatures under high irradiance. J. Japan. Soc. Hort. Sci. 64(4), 749-756. [ Links ]
Zahara, M., and H. Timm. 1973. Influence of plant density of growth, nutrient composition, yield and quality of mechanically harvested tomatoes. J. Amer. Soc. Hort. Sci. 98, 513-516. [ Links ]
Zhang, J.H., W.D. Huang, Y.P. Liu, and Q.H. Pan. 2005. Effects of temperature acclimation pretreatment on the ultrastructure of mesophyll cells in young grape plants (Vitis vinifera L. cv. Jingxiu) under cross-temperature stresses. J. Integr. Plant Biol. 47, 959-970. [ Links ]














