Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Agronomía Colombiana
versão impressa ISSN 0120-9965
Agron. colomb. v.28 n.2 Bogotá maio/ago. 2010
FISIOLOGÍA DE CULTIVOS
Dry matter accumulation and foliar K, Ca and Na contents in salt-stressed cape gooseberry (Physalis peruviana L.) plants
Acumulación de masa seca y contenido foliar de K, Ca y Na en uchuva (Physalis peruviana L.) bajo estrés por salinidad
Diego Miranda1, 3, Christian Ulrichs2, and Gerhard Fischer1
1 Department of Agronomy, Faculty of Agronomy, Universidad Nacional de Colombia, Bogota (Colombia).
2 Faculty of Agriculture and Horticulture, Division Urban Plant Ecophysiology, Humboldt-Universität zu Berlin, Berlin (Germany).
3 Corresponding author. dmirandal@unal.edu.co
Received for publication: 21 December, 2010. Accepted for publication: 28 July, 2010.
ABSTRACT
A pot experiment aimed at determining the effect of five NaCl concentrations (namely 0, 30, 60, 90 and 120 mM, determining respective EC values of 0.8, 3.0, 6.0, 9.0 and 12.2 dS m-1) on cape gooseberry plants was set up at Humboldt University's greenhouse in Berlin, Germany. Dry weight (DW) of roots, stems and leaves, as well as foliar content of ions K+, Ca2+ and Na+ and the relationship they hold with one another, were determined over a 75-day period. DW of all plant organs was observed to decrease with increasing salinity. The lowest values of this variable, which were recorded from the 120 mM NaCl plants, were found to be significantly smaller than those recorded at 60 mM and lower salt concentrations. Salt stress effects on dry matter (DM) accumulat ion were observed to increase with plant age. Leaf K+ content increased with salinity and peaked at 90 mM NaCl, where the value was significantly higher than that observed at 120 mM. Foliar Ca2+ content remained unchanged at the different salt concentrations, whilst Na+ content increased together with salt stress. The relationship among ion concentrations was significantly influenced only by 90 mM or higher NaCl concentrations, which determined a progressive increase of the Na+/Ca2+ ratio and a similar decrease of the K+/Na+ ratio. According to the results, cape gooseberry can be considered as moderately tolerant to salt, as shown by the 30 mM NaCl treatment, which did not affect DM accumulation in plant organs. This tolerance is also supported by steady leaf Ca2+ contents at all levels of salinity, indicating that cape gooseberry uses K+ as an osmoprotectant, at least up to 90 mM NaCl.Key words: dry weight, Na+/Ca2+ ratio, K+/Na+ ratio, salt-tolerant, osmoprotectant.
RESUMEN
Con el fin de determinar el efecto de cinco concentraciones de NaCl (0, 30, 60, 90 y 120 mM, las cuales determinan valores de conductividad eléctrica de 0,8; 3,0; 6,0; 9,0 y 12,2 dS m-1, respectivamente) sobre plantas de uchuva, se llevó a cabo un experimento en el invernadero de la Universidad de Humboldt (Berlín, Alemania). Durante un periodo de 75 días se evaluó el peso seco (DW) de las raíces, tallos y hojas, y el contenido foliar de K+, Ca2+ y Na+, así como las relaciones que estos iones mantienen entre si. DW disminuyó con el aumento de la salinidad, mostrando los menores valores a la concentración NaCl 120 mM. Estos valores fueron significativamente más bajos que los encontrados a 60 mM y a concentraciones menores. Se pudo constatar que la edad de la planta afectó la acumulación de DW como respuesta al estrés salino. El contenido foliar de K+ aumentó con la salinidad y fue significativamente mayor a la concentración NaCl 90 mM que a 120 mM. A los diferentes niveles de salinidad estudiados, el contenido foliar de Ca2+ se mantuvo constante, mientras que el de Na+ registró un aumento. Las relaciones entre los contenidos iónicos fueron significativamente diferentes a concentraciones iguales o superiores a NaCl 90 mM, las cuales determinaron un progresivo aumento de la relación Na+/Ca2+, y un decremento similar en la relación K+/Na+. Dado que a la concentración NaCl 30 mM no se afecta la acumulación de DW, los autores consideran que la uchuva es moderadamente tolerante a la salinidad. Esta tolerancia es respaldada por el contenido foliar de Ca2+, el cual permaneció estable a los diferentes niveles de salinidad que fueron evaluados, indicando que la planta de uchuva usa el K+ como osmoprotectante, por lo menos hasta la concentración NaCl 90 mM.
Palabras clave: peso seco, relación Na+/Ca2+, relación K+/Na+, tolerante a la sal, osmoprotectante.
Introduction
Salinity problems occur in semiarid and arid regions where the amount of rainfall is insufficient for substantial leaching of salt from the soil (Marschner, 2002), and in intensively (ferti)irrigated agricultural areas where drainage is insufficient (Larcher, 2002). It has been estimated that one-third of irrigated lands worldwide is being significantly affected by salinity (Tester and Davenport, 2003).
Marschner (2002) clearly established that nutrient misbalance is the greatest limitation for plant growth in saline substrates, due to uptake and/or shoot transport restrictions and unequal mineral nutrient distribution. Taiz and Zeiger (2006) described injury effects of salt stress as a) osmotic ones, determined by dissolved solutes in the root zone, which lower osmotic potential and consequently reduce soil water potential; and b) those resulting from ion toxicity when injurious concentrations of, for example, Na+, Cl-, or SO42-, accumulate in the cells.
The factors that influence a plant's response to a given level of salinity include ion concentration and composition in the soil solution and plant genotype (Cramer, 2004). Salts in soil act in concert with their effects on plants, rather than independently. Such is the case of the interactions between Na+ and Ca2+ (Tester and Davenport, 2003). The Na+ concentrations in plants grown in saline soils are usually higher than those of K+ or Ca2+, due to passive accumulation of Na+ in roots and shoots (Bohra and Doerffling, 1993). Elevated Na+ levels tend to displace Ca2+ from root membranes, which in turn change their integrity, and consequently their selectivity for K+ uptake from the external solution (Cramer et al., 1985). As the absorption of this ion regulates its loading into the system (Engels and Marschner, 1992), Na+ salinity is likely to determine lower shoot and higher root K+ contents, because in addition to reducing K+ uptake rate, Na+ also interferes considerably with K+ translocation from root to shoot, as confirmed by Kant and Kafkafi (2002). Salinity-related increments in Na+ content and decrements in K+ and Ca2+ contents, and in K+/Na+ and Ca2+/Na+ ratios in plants have been extensively reported in the literature (e.g., Ruiz et al., 1997; Cramer et al., 1985).
Biomass production in plants provides reliable criteria for assessing of salinity stress since growth processes are especially sensitive to excessive salt applications (Larcher, 2002). Leaves are more vulnerable to Na+ than roots because the latter accumulate lower Na+ and Cl- concentrations than the shoots (Tester and Davenport, 2003).
With regards to the experimental control of salinity, the use of solution cultures under greenhouse and laboratory conditions –where mineral concentrations are uniform in space and time– has led to a better understanding of salt-tolerance and physiological mechanisms of nutrient uptake and discrimination in crops (Grattan and Grieve, 1999).
In Colombia, cape gooseberry (Physalis peruviana L., Solanaceae) is one of the most important export fruits, and is partially grown in soils where NaCl levels are relatively high (EC > 4 dS m-1). On these grounds, the current research study aimed to determine the effect of increasing NaCl concentrations in the substrate on foliar K+, Ca2+ and Na+ contents, with regards to vegetative growth response expressed as organ dry matter accumulation.
Materials and methods
The experiment was carried out in a glass greenhouse at the Faculty of Agriculture and Horticulture of Humboldt University in Berlin, Germany (13°30' N, 52°30' W), between April and July of 2007. Forty five-day-old seed-obtained plants of the Colombia ecotype of P. peruviana were transplanted to black plastic pots (2 L) containing perlite as substrate, where they grew over a period of 75 d. The climatic conditions in the greenhouse during the experiment were 23°C mean air temperature and 60.2% mean relative humidity.
The plants were staked with a polypropylene fiber, but not pruned. The pots were placed in double rows on elevated tables inside the greenhouse, at a 0.5 m distance between rows, and 0.35 m between plants, resulting in a density of 5.7 plants/m2. Before starting the experiment, the plants were supplied with a commercially formulated complete fertilizer.
A 0.2 g L-1 nutrient solution of KristalonTM (19-6-20-3: N, P, K, Ca + micronutrients) in water was prepared for the adult cape gooseberry plants. Water electrical conductivity (EC) was 0.8 dS m-1, while the nutrient solution registered a value of 1.4 and an average pH of 5.2. The solution was applied with a Dosatron connected to a humidity sensor in the substrate, at a rate of 0.5% in two daily applications taking three minutes each.
Over a period of 75 d, five treatments of 0, 30, 60, 90 and 120 mM NaCl were evaluated in plots containing 68 plants each, with three repetitions per treatment. The saline solutions were applied to the plants every two days using a 500 mL beaker. Four samplings were carried out at 45, 55, 65 and 75 d after transplanting (dat). At 45, 55, 65 and 75 dat, three randomly selected plants per treatment and replication were chosen for root, leaves, stems and total DW determination. The samples were dried at 70°C for 48 h.
For mineral content determination, 250 mg of foliar samples were taken from the medium third of each plant and its branches. The samples were washed in deionised and bidistilled water and placed in 0.1 N HNO3 for a week. Ca2+, K+ and Na+ contents were determined using atomic absorption spectrometry (Perkin Elmer Model AAS 4100).
Irrigation water EC was measured directly twice a week using a pH conductivimeter (Hanna Instruments, HI 9811). After temperature adjustment, the average results of all the measurements taken along the experiment were 0.79 (non-saline control), 3.94 (30 mM NaCl), 6.36 (60 mM NaCl), 9.62 (90 mM NaCl) and 12.53 dS m-1 (120 mM NaCl). The pH of the different solutions was also recorded, averaging 7.90, 7.96, 8.06, 8.11 and 8.12, respectively.
The data were analyzed using SAS 9.1 software Anova procedures; mean comparison was carried out through Tukey's test (P=0.05).
Results and Discussion
Plant dry weight
Vegetative growth and plant organ DM have been reported as the most commonly used criteria for identifying salt-tolerance (Kepenek and Koyuncu, 2002). According to our results, root, stem, leaf and total DW increased with crop age, showing differences between NaCl treatments (Tab. 1). During the first 45 d, root DW accumulation did not differ between salt treatments, whereas stem DW accumulation was not-affected until day 35. However, leaves and total DW accumulation were significantly reduced by increasing NaCl concentrations from 0 to 120 mM since the first sampling date. The lowest leaf DW at the highest NaCl concentration. This could be due to decreased leaf area expansion as a water saving measure (Dodd and Davies, 2004) to compensate for the fact that salt-stressed plants absorb less water than non-stressed ones (Marschner, 2002). As the experiment progressed, in most cases the highest salt concentrations (90 and 120 mM NaCl) significantly decreased accumulated DM as compared to lower salt levels. The fundamental effects of sodium chloride on morphophysiological indicators have to do with ion toxicity, which determines consequent reductions in growth and mitotic cell division (Casierra-Posada, 2006; Mohamedin et al., 2006), as well as in water absorption and metabolic activity (Ghoulam et al., 2002). Similarly, Ion interference has been observed to produce nutrient deficiencies, which in turn trigger additional limitations (Grattan and Grieve, 1999). Inhibited nutrient uptake, transport and utilization by the plant as a result of high salinity-stress probably contribute to growth reduction as well (Marschner, 2002).
Due to the lack of statistical differences in DM accumulation between 0 and 30 mMol NaCl, cape gooseberry can be classified as moderately tolerant to salinity when compared to other Solanaceae fruit species such as lulo (Solanum quitoense L.) (Ebert et al., 1999; Flórez et al., 2008) and tomato (Solanum lycopersicum L.) (Cuartero and Fernández-Muñoz 1999). Similarly, Bergmann (1993) classified tomato, which is a relative of cape gooseberry, as well tolerant to salinity.
Ion content
Potassium
Slightly affected by increasing NaCl content, potassium levels in the studied cape gooseberry leaves reached a peak at about 60 to 90 mM NaCl (Tab. 2). From days 55 to 75, they were significantly lower at the highest salt concentration than at 90 mM NaCl. Interestingly, however, 0 and 120 mM NaCl determined nearly the same average leaf K+ content (Tab. 2).
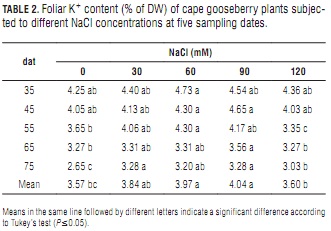
Several horticultural crop studies have shown that K+ concentration in leaf tissue decreases with increasing salinity (e.g., Grattan and Grieve, 1999; Miranda et al., 2008). Ebert et al. (1999) found that foliar K+ content was reduced significantly from 0 through 30 to 60 mM NaCl in lulo. Also, Bayuelo-Jiménez et al. (2003) described severely reduced K+ root uptake rates at increased salinity (0 to 80 mM NaCl). In contrast, the present work registered a tendency to accumulate K+ with increasing salt concentration (up to 90 mM). This result coincides with data reported by Cachorro et al. (1993), who found that K+ levels in the cells of bean leaves increased with increasing NaCl-salinity.
This could indicate either a compensation effect through K+ re-translocation from roots and stems to leaves (Romero et al., 1994), or a sustained acquisition persisting in spite of higher Na+ uptake (Lynch et al., 1982). Additionally, K+ can act as a compatible inorganic solute favoring the osmotic adjustment process that allows the plant to withstand hyperosmotic ion stress (Taiz and Zeiger, 2006). Probably because of increasing leaf injuries at 120 mM NaCl (Miranda et al., 2010), this osmoprotectant function was less effective at this salinity-stress level.
Also, the adequate Ca2+ level in the substrate could have influenced K+/Na+ selectivity by shifting the uptake ratio in favor of K+ at the expense of Na+ (Grattan and Grieve, 1999), which partially agrees with the finding that there were no significant mean K+/Na+ ratio differences between 0 and 60 mM NaCl.
Calcium
In general, Calcium content was observed to decrease slightly as the plant age (Tab. 3). Leaf Ca2+ content was only slightly affected by the salinity treatments until the third sampling date, when it started to show significant differences. Paralleling K+ behavior, from 55 to 75 dat, calcium content was significantly lower at 120 than at 90 mM NaCl (Tab. 3). The lower potassium levels recorded at 120 mM NaCl after day 55 indicate that high salinity probably alters Ca2+ uptake and transport within the plant (Bayuelo-Jiménez et al., 2003). Also, high leaf Na+/Ca2+ ratios (Tab. 5) at this salt concentration may represent restricted Ca2+ transport from the roots. Most of all, it must be highlighted that with increasing salt concentration in the root zone, there is a concomitant increment in the plant's requirement for Ca2+ (Grattan and Grieve, 1999).
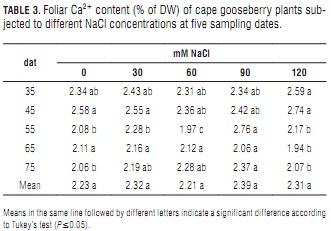
The absence of significant differences between mean Ca2+ concentrations indicates that salt stress was not intense enough to considerably affect membrane permeability. A similar behavior has been reported by Lycoskoufis et al. (2005) in salinized pepper plants. Bergmann (1993) described a reduced Ca2+ uptake due to excessive Na+ application, but this effect was not pronounced in cape gooseberry at the salt concentrations studied in the present work. Moreover, external Ca2+ facilitates K+ and minimizes Na+ uptake (Taiz and Zeiger, 2006) through the closure of specific Na+ transport channels (Rus et al., 2001). Sodium
In plants with a fair degree of tolerance to sodicity, Na+ substitutes K+ in nonspecific homeostatic functions (Epstein and Bloom, 2005). Leaves being the preferred organs for Na+ accumulation (Tester and Davenport, 2003), it was no surprise to see it increase with NaCl concentration in the present experiment. At 120 mM NaCl, Na+ content was significantly different from those at lower salt concentrations (Tab. 4). Increasing foliar Na+ concentrations have been directly tied to increasing NaCl concentrations in irrigation water (Casierra-Posada et al., 2000; Carter and Grieve, 2008). In general, Na+ content was found to decrease along the experiment, such behavior being more pronounced at low and zero salt applications.
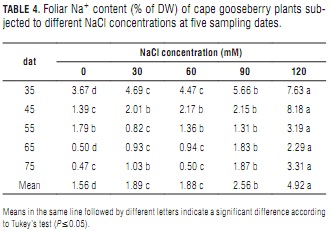
Besides the dilution effect that results from plant aging, reduced Na+ concentration at late sampling dates could indicate a certain ability of cape gooseberry to reduce sodium transport from root to shoot through Ca2+ activity. Assuming that Ca2+ is directly involved in Na+ exclusion and retention mechanisms, Melgar et al. (2006) proposed that the former ion could act as an inhibitor (and therefore a regulator) of Na+ transport from root to shoot in olives. This could be one of the plant's most vital abilities for surviving in low saline conditions.
The toxicity symptoms of older leaves (yellowing, marginal necrosis and final abscission) observed in the 120 mM NaCl treated plants are consistent with those described in the literature for salt stress, specifically regarding Na+ toxicity (Bergmann, 1993; Tester and Davenport, 2003). Leaf abscission is a mechanism for preventing excessive solute buildup in the cell wall water (Nobel, 1999).
Na+/Ca2+ ratio
The rising Na+ (Tab. 4) and nearly constant Ca2+ concentrations (Tab. 3) that accompanied increasing salinity determined concomitant Na+/Ca2+ ratio increments (Tab. 5), which were more pronounced with increasing plant age. At day 75, the leaves of the plants treated with the 120 mM NaCl solution had an 87% higher ratio than the control plants. As in the case of lulo (Ebert et al., 1999), the plants tended to maintain a low Na+/Ca2+ ratio. Because Ca2+ content is associated to ion-selectivity of membranes (Marschner, 2002), maintaining an appropriate Na+/Ca2+ balance is vital for non-halophytes to survive in a salt-affected environment (Grattan and Grieve, 1999). Cramer (2004) concluded that growth, photosynthesis, plant nutrition and water and ion transport in plants are affected by plant genotype-modulated Na+-Ca2+ interactions.
The effect of the significantly higher Na+/Ca2+ ratios found in the leaves of the plants treated with 90 and 120 mMol NaCl as compared to those resulting from lower salt concentrations (Tab. 5) is reflected in their lower leaf DW (Tab. 1). Bernstein et al. (1993) observed a leaf length reduction in sorghum plants treated with 100 mM NaCl. This shortening was prevented by increasing Ca2+ concentration in the nutrient solution from 1 to 10 mM. The premature senescence of these older leaves was most probably the result of Na+ toxicity at high Na+/Ca2+ ratios and Ca2+ toxicity at low Na+/Ca2+ ratios. Calcium toxicity may also be a factor in the reduction of shoot growth at high external Ca2+ levels (Yamanouchi et al., 1983).
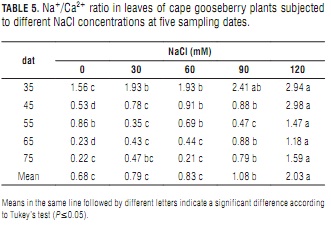
Na+/Ca2+ ratio decrease with increasing plant age may be indicating that the latter promotes a growing Na+ tolerance that hampers the excessive accumulation of this mineral observed in foliar tissues. A similar situation was reported in tomato by Pérez-Alfocea et al. (1996), who observed that salinity reduced K+, Ca2+ Mg2+ and NO3- foliar concentrations, and that plants absorbing more of these nutrients from the medium could have much lower Na+/Ca2+ ratios and a nutrient equilibrium similar to that of non-salinized plants, especially in younger leaves undergoing active development.
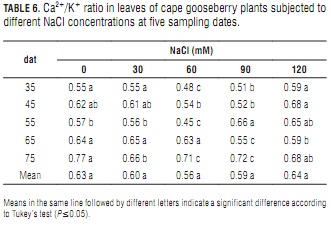
Ca2+/K+ ratio
In general, NaCl salt-stress did not cause significant changes in mean leaf Ca2+/K+ ratio (Tab. 6). However, in three of the five sampling dates, this ratio was higher at the highest salt level (Tab. 6). This indicates that the plants tended to maintain a stable Ca2+ content at this elevated salt level, whereas K+ did not withstand.
K+/Na+ ratio
K+/Na+ ratios decreased significantly with increasing NaCl concentrations, this effect being more pronounced with plant aging (Tab. 7). At day 75, 120 mM NaCl treated plants had an 84% lower ratio than the control plants, whereas it was only 51% at day 35.
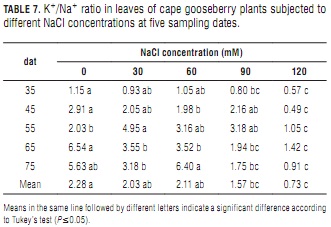
Decreasing K+/Na+ ratios have also been found by Yildiz et al. (2008) in strawberry and by Ebert et al. (1999) in lulo, among others. Ioneva (1988) attributed foliar K+/Na+ ratio reductions to increased Na+ contents resulting from competition between Na+ and K+ ions at plant root absorption sites. Ashraf and McNeilly (2004) suggested that constantly high K+/Na+ tissue ratios feature salt-tolerance in Brassica sp. Sodium induced K+ deficiency has been implicated in abnormal growth and reduced yield in several crops including tomato (Song and Fujiyama, 1996; López and Satti, 1996), spinach (Chow et al., 1990), rosemary (Tounekti et al., 2008), fennel (Graifenberg et al., 1996) and corn (Botella et al., 1997).
Kant and Kafkafi (2002) reported that besides reducing K+ uptake rate, Na+ salinity also interferes to a great extent with K+ translocation from root to shoot, which results in lower shoot and higher root K+ contents. In working with maize seedlings, Botella et al. (1997) observed that the inhibitory effect of salinity on K+ translocation was stronger at low K+ concentrations in the nutrient solution.
Conclusions
The present research study suggests that cape gooseberry is moderately tolerant to salt, as it can be seen in the 30 mM NaCl treated plants, whose organs showed no DM accumulation differences with the control plants. There is some evidence, indicated by the increasing foliar K+ concentrations with increasing NaCl salinity (up to 90 mM), that cape gooseberry plants use potassium as a compatible solute for osmotic adjustment.
Cited references
Ashraf, M. and T. McNeilly. 2004. Salinity tolerance in Brassica oil seeds. Critical Rev. Plant Sci. 23, 157-174. [ Links ]
Bayuelo-Jiménez, J.S., D.G. Debouck, and J.P. Lynch. 2003. Growth, gas exchange, water relations, and ion composition of Phaseolus species grown under saline conditions. Field Crops Res. 80, 207-222. [ Links ]
Bergmann, W. 1993. Ernährungsstörungen bei Kulturpflanzen. 3. Auflage. Gustav Fischer Verlag, Jena, Germany. [ Links ]
Bernstein, N., A. Läuchli, and W.K. Silk. 1993. Kinematics and dynamics of sorghum (Sorghum bicolor L.) leaf development at various Na+/Ca2+ salinities. I. Elongation growth. Plant Physiol. 103, 1107-1114. [ Links ]
Bohra, J.S. and K. Doerffling. 1993. Potassium nutrition of rice (Oryza sativa L.) varieties under NaCl salinity. Plant Soil 152, 299-303. [ Links ]
Botella, M.A., V. Martinez, J. Pardines, and A. Cerda, A. 1997. Salinity induced potassium deficiency in maize plants. J. Plant Physiol. 150, 200-205. [ Links ]
Cachorro, P., A. Ortiz, and A. Cerda. 1993. Growth, water relations and solute composition of Phaseolus vulgaris L. under saline conditions. Plant Sci. 95, 23-29. [ Links ]
Carter, C.T. and C.M. Grieve. 2008. Mineral nutrition, growth, and germination of Antirrhinum majus L. (snapdragon) when produced under increasingly saline conditions. HortScience 43(3), 710-718. [ Links ]
Casierra-Posada, F. 2006. Distribución y producción total de materia seca en guayabo (Psidium guajava L. cv. Palmira ICA-1) bajo estrés salino. Orinoquia 10(2), 59-66. [ Links ]
Casierra-Posada, F., G. Ebert, and P. Lüdders. 2000. Efecto de la salinidad por cloruro de sodio sobre el balance de nutrientes en plantas de lulo (Solanum quitoense L.). Agron. Colomb. 17, 85-90. [ Links ]
Chow, W.S., M.C. Ball, and J.M. Anderson. 1990. Growth and photosynthetic responses of spinach to salinity: Implications of K. nutrition for salt tolerance. Aust. J. Plant Physiol. 17, 563-578. [ Links ]
Cramer, G.R. 2004. Sodium-calcium interactions under salinity stress. pp. 205-228. In: Läuchli, A. and U. Lüttge (eds.). Salinity: Environment-plants-molecules. Kluwer Academic Publ., New York, NY. [ Links ]
Cramer, G.R., A. Läuchli, and V.S. Polito. 1985. Displacement of Ca2+ from the plasmalemma of root cells. Plant Physiol. 79, 297-211. [ Links ]
Cuartero, J. and R. Fernández-Muñoz. 1999. Tomato and salinity. Scientia Hort. 78, 83-125. [ Links ]
Dodd, I.C. and W.J. Davies. 2004. Hormones and the regulation of water balance. pp. 493-512. In: Davies, P.J. (ed.). Plant hormones: biosynthesis, signal transduction, action! Kluwer Academic Publ., Dordrecht, The Netherlands. [ Links ]
Ebert, G., F. Casierra, and P. Lüdders. 1999. Influence of NaCl salinity on growth and mineral uptake of lulo (Solanum quitoense L.). J. Appl. Bot. 73, 31-33. [ Links ]
Engels, C. and H. Marschner. 1992. Adaptation of potassium translocation into the shoot of maize (Zea mays L.) to shoot demand: Evidence for xylem loading as a regulating step. Physiol. Plant. 86, 263-268. [ Links ]
Epstein, E. and A.J. Bloom. 2005. Mineral nutrition of plants: principles and perspectives. Sinauer Associates Publ., Sunderland, MA. [ Links ]
Flórez, S.L., D. Miranda L., B. Chaves, G. Fischer, and S. Magnitskiy. 2008. Growth of lulo (Solanum quitoense Lam.) plants affected by salinity and substrate. Rev. Bras. Frutic. 30(2), 402-408. [ Links ]
Graifenberg, A., L. Botrini, L. Giustiniani, and M. Lipucci Di Paola. 1996. Salinity affects growth, yield and elemental concentration of fennel. HortScience 31, 1131-1134. [ Links ]
Grattan, S.R. and C.M. Grieve. 1999. Mineral nutrient relations in horticultural crops. Scientia Hort. 78, 127-157. [ Links ]
Ghoulam, C., A. Foursy, and K. Fares. 2002. Effects of salt stress on growth, inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Environ. Exp. Bot. 47, 39-50. [ Links ]
Ioneva, Z.S. 1988. Effect of potassium ion Na+ uptake by plants in conditions of chloride salinity. Fiziolo. Rasten. 14, 42-47. [ Links ]
Kant, S. and U. Kafkafi. 2002. Potassium and abiotic stresses in plants. pp. 233-251. In: Pasricha, N.S. and S.K. Bansal (eds.). Role of potassium in nutrient management for sustainable crop production in India. Potash Research Institute of India, Guargaon, Haryana, India. [ Links ]
Kepenek, K. and F. Koyuncu. 2002. Effect of salt expression of resistance in some domestic foreign strawberry cultivars. Acta Hort. 573, 289-295. [ Links ]
Larcher, W. 2002. Physiological plant ecology. Ecophysiology and stress physiology of functional groups. 4th ed. Springer Verlag, Berlin. [ Links ]
López, M.V. and S.M.E. Satti. 1996. Calcium and potassium-enhanced growth and yield of tomato under sodium chloride stress. Plant Sci. 114, 19-27. [ Links ]
Lycoskoufis, I.H., D. Savvas, and G. Mavrogianopoulos. 2005. Growth, gas exchange and nutrient status in pepper (Capsicum annuum L.) grown in recirculating nutrient solution as affected by salinity imposed to half of the root system. Scientia Hort. 106(2), 147-161. [ Links ]
Lynch, J., E. Epstein, and A. Läuchli. 1982. Na+-K+ relationship in salt stressed barley. pp. 347-352. In: Scaife, A. (ed.). Proc. 9th Intl. Plant Nutr. Coll., Warwick. Commonw. Agr. Bureau, Farnham Royal, Bucks, UK. [ Links ]
Marschner, H. 2002. Mineral nutrition of higher plants. Academic Press, San Diego, CA. [ Links ]
Melgar, J.C., M. Benlloch, and R. Fernández-Escobar. 2006. Calcium increases sodium exclusion in olive plants. Scientia Hort. 109, 303-305. [ Links ]
Miranda L., D., C. Carranza, and G. Fischer. 2008. Calidad de agua de riego en la Sabana de Bogotá. Universidad Nacional de Colombia, Facultad de Agronomía, Bogotá. [ Links ]
Miranda L., D., G. Fischer, and Ch. Ulrichs. 2010. Growth of cape gooseberry (Physalis peruviana L.) plants affected by salinity. J. Appl. Bot. Food Qual. 83(2) 175-181. [ Links ]
Mohamedin, A.A.M., A.A. Abd El-Kader, and N.M. Badran. 2006. Response of sunflower (Helianthus annus L.) to plants salt stress under different water table depths. J. Appl. Sci. Res. 2(12), 1175-1184. [ Links ]
Nobel, P.S. 1999. Physicochemical and environmental plant physiology. 2nd ed. Academic Press, San Diego, CA. [ Links ]
Pérez-Alfocea, F., M.E. Balibrea, A. Santa Cruz, and M.T. Estan. 1996. Agronomical and physiological characterisation of salinity tolerance in a commercial tomato hybrid. Plant Soil 180, 251-257. [ Links ]
Romero, J.M., T. Marañon, and J.M. Murillo. 1994. Long-term responses of Melilotus segetalis to salinity. I. Growth and partitioning. Plant Cell Environ. 17, 1243-1248. [ Links ]
Ruiz, D., V. Martínez, and A. Cerdá. 1997. Citrus response to salinity: growth and nutrient uptake. Tree Physiol. 17, 141-150. [ Links ]
Rus, A., S. Yokoi, A. Sharkuu, M. Reddy, B.H. Lee, T.K. Matsumoto, H. Koiwa, J.K. Zhu, R.A. Bressan, and P.M. Hasegawa. 2001. AtHKT1 is a salt tolerance determinant that controls Na+ entry into plant roots. Proc. Natl. Acad. Sci. USA 98, 14150-14155. [ Links ]
Song, J.Q. and H. Fujiyama. 1996. Difference in response of rice and tomato subjected to sodium salinization to the addition of calcium. Soil Sci. Plant Nutr. 42, 503-510. [ Links ]
Taiz, L. and E. Zeiger. 2006. Plant physiology. 4th ed. Sinauer Associates Publ., Sunderland, MA. [ Links ]
Tester, M. and R. Davenport. 2003. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 91, 503-527. [ Links ]
Tounekti, T., A.M. Vadel, A. Bedoui, and H. Khemira. 2008. NaCl stress affects growth and essential oil composition in rosemary (Rosmarinus officinalis L.). J. Hort. Sci. Biotechnol. 83(2), 267-273. [ Links ]
Yildiz, K., Ö. Uzal, and H. Yilmaz. 2008. Consequences of NaCl salinity on growth and ion accumulation in selected strawberry cultivars. Eur. J. Hort. Sci. 73(2), 69-72. [ Links ]
Yamanouchi, M., Y. Shimada, and S. Yoshida. 1983. The effects of calcium ion to reduce the toxicity of sodium chloride for rice plant. Jap. J. Soil Sci. Plant Nutr. 54, 499-504. [ Links ]




![Soybean breeding (Glycine max [L.] Merril) for the Colombian Altillanura: a prospective and conceptual view](/img/pt/prev.gif)









