Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Agronomía Colombiana
Print version ISSN 0120-9965
Agron. colomb. vol.29 no.2 Bogotá June/Aug. 2011
CROP PHYSIOLOGY
Proteomics: a tool for the study of plant response to abiotic stress
La proteómica: herramienta para el estudio de la respuesta de las plantas al estrés abiótico
Gabriel Roveda-Hoyos1, and Liz P. Fonseca-Moreno1,2
1Department of Agronomy, Faculty of Agronomy, Universidad Nacional de Colombia. Bogotá (Colombia).2Corresponding author. lpmorenof@unal.edu.co Recceived for publication: 17 February, 2010. Accepted for publication: 2 June, 2011.
ABSTRACT Due in part to human activity, changes in global climate behavior have manifested in an increase in extreme temperature related events such as drought, salinization, contamination and flooding of vast areas of the planet. Regarding agricultural activity, these uncertain climatic scenarios are likely to cause biotic and abiotic stress increases, which must be dealt with through science and technology. Holistic approaches, also known as "omics": proteomics, genomics, transcriptomics, and metabolomics offer new ways of facing these coming climate changes. Proteomics provide a new approach to the identification of proteins of interest and to carry out a functional analysis of the genome and its relationship with the environment. New advances in proteomics include the use of highly efficient techniques such as bi-dimensional electrophoresis, multi-dimensional chromatography, mass spectrometry and second generation technologies for the analysis of polypeptides and proteins at tissue, organ, organelle and membrane levels, as well as bioinformatic tools. This review article is comprised of aspects related to the general model of stress in plants, and advances in proteomics which contribute to the understanding of water and salt stress in cereals of economic importance. Key words: water deficit, salinity, omics, ABA. RESUMEN Los cambios en el comportamiento climático global, como consecuencia en parte de la actividad humana, se manifiestan con el incremento en la intensidad de eventos relacionados con temperaturas extremas, sequía, salinización, contaminación y anegación de extensas áreas del planeta. Estos nuevos escenarios climáticos de mayor incertidumbre para la actividad agrícola se relacionan con el aumento en la incidencia de estreses bióticos y abióticos, que deberán ser abordados desde el conocimiento científico y la tecnología. Las aproximaciones de carácter holístico, conocidas como "Ómicas", genómica, transcriptómica, proteómica y metabolómica ofrecen nuevas oportunidades para enfrentar los cambios climáticos venideros. La proteómica proporciona una nueva aproximación que permite identificar proteínas de interés y realizar un análisis funcional del genoma y de su relación con el ambiente. Los nuevos avances en proteómica incluyen el uso de tecnologías de alta eficiencia como la electroforesis bidimensional, la cromatografía multidimensional, la espectrometría de masas y tecnologías de segunda generación, que junto con herramientas de bioinformática permiten el análisis de polipéptidos y proteínas a nivel de tejidos, órganos, organelos y membranas. Este artículo de revisión, comprende aspectos relacionados con el modelo general de estrés en plantas y avances de la proteómica en la comprensión del estrés hídrico y salino en cereales de importancia económica.
Palabras clave: déficit hídrico, salinidad, ómicas, ABA.
Introduction
The steady increase in demand for food and the presence of abiotic stresses such as drought, salinity, floods and desertification which affect the planet, due in part to climate change, create a new challenge for modern agriculture. According to FAO data (2006 and 2011) during the 70s, the problem of malnutrition in the world affected about 850 million people, today the situation has worsened and it is estimated that by the end of 2010 with 925 million inhabitants, with 52.5 million in Latin America. This will have major implications for food demand and the supply of natural resources.
Much evidence confirms the increase of concentrations of CO2 and other greenhouse gases such as methane and nitrous oxide resulting from burning fossil fuels (IPCC, 2007). These changes are responsible for large increases in temperature, observed in the second half of the twentieth century, as reported by the International Panel on Climate Changes (IPCC) (2007). Studies based on climate models predict changes in average temperatures between 4.0 and 6.4°C at the surface during the next six decades. Global warming will cause drastic changes in rainfall patterns, with significant consequences for agriculture, wildlife and life in general on the planet (WWF, 2008).
Under future climate conditions, global agricultural production is increasingly considered a high risk activity, competition for natural resources like water and agricultural land will become more critical due to increased desertification and floods (Kundzewicz et al., 2005). The increase in the intensity of events related to extreme temperatures: drought, salinization and water logging of large areas of the planet, will significantly affect agricultural production. It is estimated that the production of cereals such as maize will be reduced by 1.5% on average for each acre planted per increments of 0.8°C over the next 30 years (FAO, 2009).
Given this scenario the new agricultural revolution must consider the selection of genetic material of plants adapted to different environmental stresses, a strategy that will be an essential part in facing the problems of securing food. The identification of desirable genes related to adaptation to stress in crops of importance should be included in breeding programs (Hashiguchi et al., 2009). The study of the ecophysiological behavior of species, including the mechanisms of adaptation to different conditions of abiotic and biotic stress, will be of fundamental importance (Hashiguchi et al., 2009). Recent research related to the study of comparative proteomics between stressors and agriculturally important crops such as Oryza sativa (Agrawal et al., 2009), Hordeum vulgare (Finnie and Svensson, 2009), Triticum sativumsativum (Caruso et al., 2009) and Glycine max (Komatsu and Ahsan, 2009; Tobari et al., 2009) contribute to the proper use of genetic diversity of cultivated plants for adaption to various ecosystems (Hashiguchi et al., 2009). This article presents an updated review of proteomics with an emphasis on understanding water and salt stress, starting with a general model of stress.
General model of stress in plants
There is now strong evidence to consider various factors that cause stress in plants, with common routes for similar responses, and presenting specific effects. These observations were initially made by Selye (1936) and are known as "General Adaptation Syndrome" (GAS). This concept evolved into a term coined as co-stress (Prasad and Rengel, 1998), which means that resistance to a particular type of stress can contribute to the co-resistance to other stresses for a mechanism of resistance to multiple stresses. This condition can be very beneficial to plant growth and development, in natural conditions, plants are exposed to various adverse factors that cause stress, simultaneously or alternately, so that a stress factor can confer or increase the tolerance of the plant to different stress (Shinozaki and Yamaguchi-Shinozaki, 2007).
This system of multiple integration has been the subject of current research interest and understanding it is a pillar for a better comprehension the physiological behavior of plants and the mechanisms of adaptation to environmental changes (Shinozaki and Yamaguchi-Shinozaki, 2007). Possibly, during the process of evolutionary selection, direct responses of plants to each environmental factor (drought, cold, heat, radiation intensity, salinity, etc.) were gradually replaced by means of perception/signal transduction and gene expression regulation, which formed an adaptation strategy that was more efficient and economical in biological terms (Leshem et al., 1998).
This general model at the cellular level in response to stress, including perception, signal transduction and regulation of gene expression, allows a physical stimulus generated from a biochemical response (Goday and Pagés, 2004). The perception of stress depends on the stimulus and in the case of water deficit may be related to changes in turgor cell pressure, which is converted into a cellular signal, with the participation of secondary messengers, initiating the transduction processes which amplifies and translates the signals into the cell nucleus. There are other signaling mechanisms where plant hormones such as abscisic acid (ABA) and jasmonic acid (JA) are involved in responses to water stress, salinity and wounds (Shinozaki and Yamaguchi-Shinozaki, 2007). Among the molecules related to signal transduction pathways mediated by ABA are protein kinases, phosphates and other molecules such as phospholipids C and D, calcium binding proteins, farnesyl transferase and hydrogen peroxide (Goday and Pagés 2004). A major common response of plants, considered a co-stress, is related to the detoxification of free radicals by the antioxidant activity of enzymes such as ascorbate peroxides (APX) and Cu/Zn superoxide dismutase (SOD) by Qureshi et al. (2007). Similarly, the expression of several enzymes involved in the synthesis of compounds such as poly osmoregulatores (mannitol and sorbitol) and non-protein amino acids preserve the plant metabolism. Other common responses are associated with heat shock proteins (HSP), proteins involved in the transport of water, ions and other compounds and LEA proteins (late embryogenesis abundant proteins) that appear to have protective functions for different types of stress (Goday and Pagés, 2004).
Other strategies to adapt to various stress conditions include mechanisms such as the formation of phenols and flavonoids in the epidermis to protect the photosynthetic apparatus from UV rays, water stress or nitrogen deficiency (Schwieger et al., 1996). A response mechanism to high lighting reduces zeaxanthin in the photoreduction of the carotenoid violaxanthin (Lichtenthaler and Schindler, 1992) and in response to high light and high temperatures shows the emission of a volatile gas, isoprene, with the formation of a photochemical smog that contributes to ozone formation (Zeidler and Lichtenthaler, 2001).
Proteomics in understanding abiotic stress Water stress
Drought is a problem of great importance in world agriculture, especially in arid and semiarid regions, which represent a third of the world (Caruso et al., 2009). The availability to use water in agriculture will be a key factor in future agricultural production. Predictive models of climate change estimate that global warming will become more frequent with severe droughts (IPCC, 2007). The economic impact of water deficit in agricultural production, may cause losses close to 50% (Kreps et al., 2002), as a result of reduced photosynthesis for one. For example, water stress inhibits CO2 fixation in soybean leaves in just a few days, reducing carbon assimilation rates to almost zero (Ribas-Carbo et al., 2005).
Plants respond to water deficit through a series of processes at the physiological, cellular and molecular levels, which can lead to stress tolerance. The effect of water stress on physiological terms has been extensively researched and plant responses include stomatal closure, reduction in cell growth, decreased photosynthetic activity and increased respiration. Among the most common responses highlighted are the damage to chlorophyll, decreased antioxidant system, increase in the production of hydrogen peroxide (H2O2) and the O2-ion, lipid peroxidation, decreased photosynthesis (Qureshi et al., 2007), increased oxidative stress and alterations in cell wall elasticity (Caruso et al., 2009). At the cellular level, plants respond and adapt to a water deficit by accumulating osmolytes and synthesizing specific proteins. The comparative analysis of water stress induced on genes of Arabidopsis and O. sativa using microarrays, revealed a high degree of similarity between the two genomes at the molecular level, 73 genes were identified in O. sativa (Rabbani et al., 2003) as water stress-inducible, 51 had been reported with similar functions in Arabidopsis (Shinozaki et al., 2003). These results confirm that there are common genes that are induced during stress in species that have evolved separately for more than a million years, such as Arabidopsis and O. sativa (Shinozaki and Yamaguchi-Shinozaki, 2007).
The identified gene products induced by water stress in Arabidopsis and O. sativa can be classified into two groups. The first group encodes proteins that probably function as tolerance mechanisms to abiotic stress, such as LEA proteins and HSP to water, salt and high temperature stress, antifreeze proteins (AFP Antifreezing Proteins), which act as chaperones and proteins involved in the transport of water, ions and other compounds. In addition to enzymes involved in the biosynthesis of osmolytes (mannitol, trehalose, galactinol and raffinose), amino acids (proline), amines (glycinabetaina and polyamines) and detoxification enzymes (Timperio et al., 2008).
The second group of genes induced by water stress allows the expression of proteins involved in signal transduction pathways mediated by ABA, including protein kinases, protein phosphates and other molecules such as phospholipids, protein bound calcium and hydrogen peroxide (Umezawa et al., 2006).
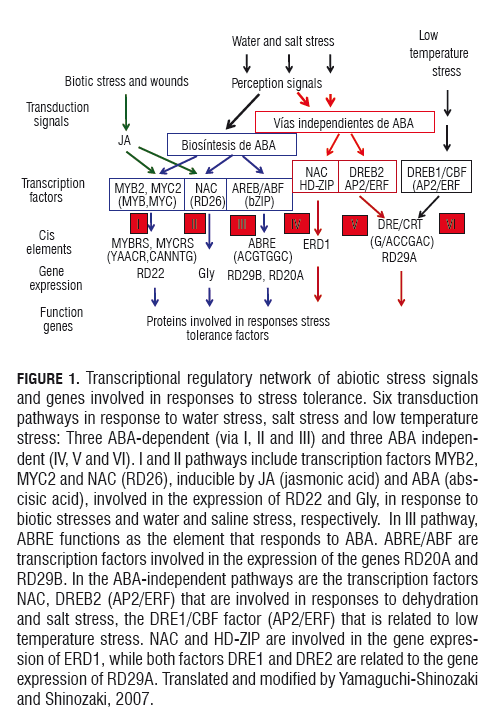
Experimental results suggest the existence of a common regulatory system in which signaling pathways are shared in the water and salinity stress induced ABA pathway, while there is a minor relationship between signaling pathways for water stress and the response to low temperatures (Rabbani et al., 2003). At least six signaling pathways have been reported by Shinozaki and Yamaguchi-Shinozaki (2007) in the activation of genes induced by conditions of water stress, salt stress and low temperature stress. Three of which are dependent on ABA (Tracks I, II and III), and three independent pathways IV, V and VI (Fig.1).
The water and saline stress depend on ABA (pathways I, II and III) inducing genes that encode transcription factors, MYB, MYC, NAC and RAEB/ABF. The first factors, such as MYB (ATMYB2) and MYC (rd22BP1), which function in the regulation of ABA- RD22 inducible gene in Arabidopsis, have conservation motives (Abe et al., 1997; Shinozaki and Yamaguchi-Shinozaki, 2007). Recently, it was found that the NAC transcription factor encoded by the RD26 inducible gene is drought and salinity based (Fujita et al., 2004). Two transcription factors of the leucine zipper type, RAEB/ABF, can bind to ABRE (ABA-responsive element) to activate the expression of RD20A and RD29B (Choi et al., 2000; Uno et al., 2000).
Water stress is not only mediated by an ABA-dependent pathway, but also by an ABA-independent pathway. NAC transcription factors and genes involved in DREB2B induce tolerance to drought and salinity on the IV and V tracks and in DREB1/CBF related to low temperature stress (via VI) independent of ABA. A conserved sequence of 9 bp, TACCGACAT, known as a response to drought (DRE),is required for the regulation of RD29A gene induction in water stress by drought and low temperature stress in a pathway independent of ABA (via V) (Shinozaki and Yamaguchi-Shinozaki, 2007). The factors that interact with the DRE sequence were detected in nuclear extracts prepared from dehydrated Arabidopsis plants. Over expression of the DREB2A transcription factor, a member of the DREB gene family, produces a degree of drought tolerance in Arabidopsis with over-regulation of many genes related to water stress and those encoding HSPs. Plants over-expressing the gene DREB2B showed high tolerance, suggesting that DREB2A is a central factor for the multiple signaling pathway (Sakuma et al., 2006).
Multiple efforts to clarify the operation of the integrated response system of plants to water stress are being made by analyzing the proteome in species such as upland rice (Rabello et al., 2008; Ke et al., 2009), wheat (Hajheidari et al., 2007; Caruso et al., 2009), maize (Álvarez et al., 2008) and melon (Yoshimura et al., 2008). The results of proteomics research in plants with different drought tolerance could be used in breeding plans.
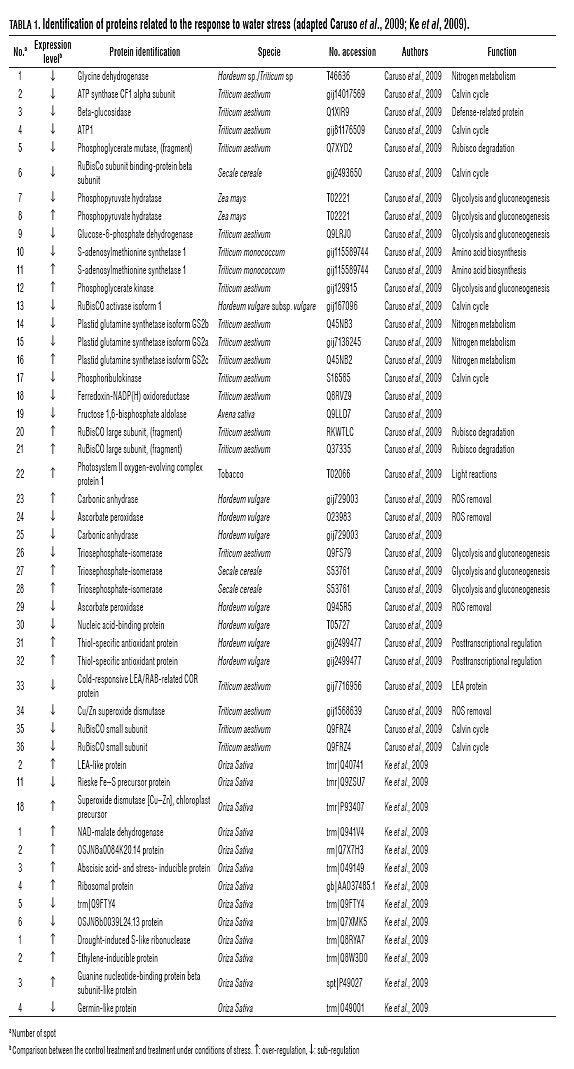
Recent research on proteomics in wheat (Triticum sativumdurum) (Caruso et al., 2009) and rice (O. sativa) (Ke et al., 2009) under water stress conditions, using the techniques of 2-DE and mass spectrometry, MALDI-TOF, show changes in protein expression, 36 to 18 for wheat and rice, with 12 proteins over-regulated and 24 sub-regulated in wheat, and 12 over-regulated and 6 sub-regulated in rice. In the case of T. durum, 36 reproducible protein spots were detected in response to water stress. This study identified 21 different proteins, including some isoforms and subunits of enzymes that change their expression under water stress. Eighteen percent of the proteins identified were related to the "primary" metabolism, particularly with the routes of glycolysis and gluconeogenesis, demonstrating that the primary metabolism can be modulated to establish a new homeostasis under water stress. Fifteen percent of the proteins were associated with the removal of reactive oxygen species (ROS), 12% amino acid biosynthesis, 9% in the Calvin cycle, 6% with defense mechanisms and the remaining 3% related to post-transcriptional regulation, these biological mechanisms are involved with drought stress in plants (Caruso et al., 2009) (Tab.1).
Water stress can affect homeostasis and cause severe toxic effects in plants through complex mechanisms that have not yet been fully characterized. The water deficit stress can affect the photosynthetic activity more than other types of abiotic stress, possibly this involves the synthesis and degradation of enzymes related to the light-dependent reactions and Calvin cycle (Caruso et al., 2009). In research done by Caruso et al. (2009), they found a differential response to water stress of six proteins involved in photosynthesis, including Rubisco isoforms (1, 5-bisphosphate carboxylase/ oxygenase), a protein involved in photosystem II (PS II), two proteins related to ATP synthesis and fosforibulosa kinesis involved in the Calvin cycle.
Other effects of water stress on the proteome of wheat are the biosynthesis of enzymes related to ROS detoxification mechanisms (ascorbate peroxidase-APX, Cu/Zn superoxide dismutase-SOD, carbonic anhydrase) and enzymes involved in proline biosynthesis (S-adenosylmethionine synthesize and glutamine synthesize), involved in osmoprotection and osmoregulation. Additionally, we found a protein involved in the pathogen defense reaction (ß-glucosidase) (Caruso et al., 2009).
The identification of differential expression of proteins and phosphoproteins induced by water stress in O. sativa, using a proteomic approach, allowed the detection of 18 proteins with changes in expression (Ke et al., 2009). We found three proteins related to the chloroplast, an LEA and SOD that were over-regulated, whereas the protein precursor of Rieske Fe-S was under-regulated, the latter may be involved in reducing rates of photosynthesis. Of the ten phosphoproteins identified in response to drought, seven had not been previously reported under conditions of water stress. These results suggest the involvement of currently unidentified proteins in the mechanisms that regulate responses to water stress (Ke et al., 2009). Protein phosphorylation is considered an important signaling mechanism of environmental stress, and is one of the most important post-translational modifications (PMT) that modulate the activity of proteins, protein-protein interaction and cellular localization (Khan et al., 2005).
Saline stress
The salinity of the soil by the presence of toxic levels of sodium, chloride and sulfate, representing 800 million hectares (ha) in the world (6% of total cultivated land), and an estimated 397 million ha are associated with sales and 434 million ha to sodium (FAO, 2005). Abiotic stress, including salinity, has reduced agricultural production in the world by over 50% (Bray et al., 2000).
Salts in the soil inhibit plant growth for two reasons: first, by an osmotic effect that reduces the capacity of plant water uptake and causes a slow plant growth as a result of water stress in response to salinity of the external environment, and second, by the toxic effect due to the entry of salts that cause tissue damage of the leaves (Khan et al., 2007). The probable cause of damage is that the high flux of ions and salts exceeds the ability of the cell to compartmentalize them in the vacuole, with the rapid entry of salts and ions into the cytoplasm, enzyme activity is inhibited, the cell wall is altered and the cells become dehydrated and die (Munns, 2005).
Plants under saline conditions generally have an osmotic imbalance, which causes a nutritional imbalance. The osmotic imbalance mainly causes changes in ion concentration in the cytoplasm, particularly potassium and calcium, to be replaced by higher levels of Na+ and Cl-, which have toxic effects on the membrane structure and the enzyme systems (Ashraf and Harris, 2004). The latter effect is related to a secondary stress, such as oxidative stress caused by the production of toxic reactive oxygen which in turn leads to lipid peroxidation (LP) production (Qureshi et al., 2005).
Several studies related to the comparative analysis of proteome between plants subjected to salt stress and control treatments have been performed in species like rice (O. sativa) (Abbasi and Komatsu, 2004; Yan et al., 2005), wheat (Triticum sativumsp.) (Huo et al., 2004), sorghum (Sectarian italics L.) (Veeranagamallaiah et al., 2008) and A. thaliana (Ndimba, 2005), using the techniques of 2-DE and mass spectrometry, MALDI-TOF/TOF-MS. The results show changes in expression of proteins related to the response to salt stress, 1,100 proteins were detected in rice, including 34 over-regulated proteins and 20 sub-regulated, while 175 proteins were detected in sorghum, most of them overregulated (Veeranagamallaiah et al., 2008).
Salt stress causes changes in more than 1,100 proteins of the proteome of roots in rice var. Nipponbore. Twelve different proteins have been identified using the methodology of peptide fingerprint identification by MS and searching databases (Yan et al., 2005). Three of these proteins were identified as enolase, four of them were previously confirmed as proteins in salt stress response, and the remaining six were new proteins involved in regulating metabolism energy, nitrogen and carbon in the removal of ROS and the stability of the cytoskeleton. This study gave further signs of the responses to salinity in rice roots and showed the extent of the proteomic approach in studies of stress in plants (Yan et al., 2005).
In the proteome analysis of mutant plants in tolerant (RH8706-49) and susceptible (H8706-34) to salinity wheat, five proteins located in the chloroplast, were identified as an ATPase transporter H+, a glutamine synthetic 2, a precursor protein (33kDa) involved in photosystem II and Rubisco (1.3-bisphosphate carboxylase/oxygenase) (Huo et al., 2004). These proteins probably play a crucial role in maintaining chloroplast function in plants under salt stress in leaves and roots of three cultivars of rice of the subspecies indica, Nipponbare, IR36 and Pokkali (Abbasi and Komatsu, 2004). Eight proteins demonstrated over-regulation in leaves in response to 50 mM NaCl for 24 h. These proteins were identified as LSY081, LSY262 and LSY363, while five proteins were identified as fructose bisphosphate aldose, a protein complex of PS II, a protein 2 (OEE2) (oxygen- Evolving enhancer protein 2) and superoxide dismutase (SOD). The latter enzyme has been reported in response to drought, low temperatures, salinity and ABA, whereas the expression of LSY081, LSY363 and OEE2 is increased by salt stress and ABA. LSY262 was expressed in leaves and roots, the aldose bisphosphate and two proteins related to PS II were expressed in leaf veins and sheath. LSY363 expressed in veins, but was not detected in the leaf sheath and not in the root. These results indicate that specific proteins are expressed in specific organs of rice plants,suggesting a coordinated response to salt stress (Abbasi and Komatsu, 2004).
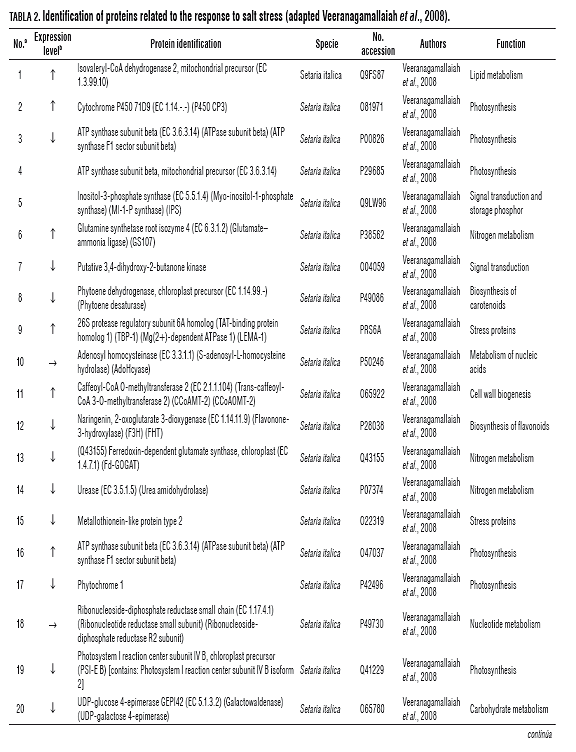
Furthermore, comparative proteome analysis of sorghum under different sodium chloride concentrations (100, 150 and 200 Mm) and control treatments showed temporal changes in total protein profile (Veeranagamallaiah et al., 2008). The results showed 175 reproducible and detectable protein spots. Through MS analysis, 29 different proteins were identified that are expressed in response to salt stress, involved in various processes such as photosynthesis (31.0%), nitrogen metabolism (13.8%) lipid metabolism (6.8%), carbohydrate metabolism (6.8%), nucleotide metabolism (6.8%), cell wall biogenesis (6.8%) and signal transduction (10.3%), stress-related proteins (10.3%) and proteins with unknown functions (7.4%). The first group represented by proteins of photosynthesis (31%) and nitrogen metabolism (13.8%), includes enzymes such as cytochrome P450 71D, phytochrome 1, proteins associated with photosystem I (PS I), chloroplast precursor (PSI.EB and EC 1.14.99) and ATP synthesis among others. In the second group, enzymes include those related to nitrogen metabolism such as glutamine synthetic, an isoform at the roots of sorghum (EC 6.3.1.2), urease (EC 3.5.1.5) and glutamate synthesis dependent ferredoxin (EC 1.4.7.1) (FD-GOGAT) (Veeranagamallaiah et al., 2008) (Tab.2).
Conclusions and perspectives
Proteomics has proved to be a valuable tool to identify proteins involved in abiotic stress responses in plants, allowing functional genome analysis. Proteins have been identified as common mechanisms of response to various abiotic stress factors (water and salt), which are expressed in different parts of the cell, such as enzymes related to the removal of ROS and protein heat shock (HSP), HSP70 is particularly common in water and salt stress. Specific response mechanisms to stress have also been identified, such as the expression of proteins involved in the synthesis of osmolytes, aquaporins and LEA proteins related to water stress.
Therefore, this omic approach contributes to the understanding of the complex mechanisms of plant response to environmental factors. Comparative proteomic studies on different tissues, organs, organelles and membranes, using different biological models (mutant or transgenic plants) allow monitoring of protein expression during different times, contributing significantly to the understanding of the mechanisms of adaptation of plants under certain stress conditions. Other research on protein-protein and proteinligand interactions and advances in approaches such as genomics, transcriptomics and metabolomics, will help to establish networks of interaction between genes, proteins and metabolites involved in stress response mechanisms. With the goal to improve protein extraction techniques in plant tissues, considered to be recalcitrant, reduce the effect of abundant proteins such as Rubisco, which hinder the display of other proteins of interest and resolve proteins with pI (s) ends. Quantitative studies of protein may increase with the development of new methodologies, known as second generation proteomics, which solve some limitations associated with the analytical variability of the technique, allowing the attainment of results with greater reproducibility, in stages of development and protein comparison of organs and between genotypes. The improvement of new computing platforms will support the rapid advancement and exchange of information between different groups in proteomics research worldwide.
Cited literature
Abbasi, F. and S. Komatsu. 2004. A proteomic approach to analyze salt responsive proteins in rice leaf sheath. Proteomics 4, 2072-2081. [ Links ]
Abe, H.K., T. Yamaguchi-Shinozaki, K. Urao, T. Iwasaki, D. Hosokawa, and K. Shinozaki.1997. Role of Arabidopsis MYC and MYB homolog's in drought- and abscísico acid regulated gene expression. Plant Cell 9, 1859-68. [ Links ]
Agrawal, G.K., N.S. Jwa, and R. Rakwal. 2009. Rice proteomics: ending phase I and the beginning of phase II. Proteomics 9, 935-963. [ Links ]
Álvarez, S., E.L. Marsh, S.G. Schroeder, and D.P. Schachtman. 2008. Metabolomic and proteomic changes in the xylem sap of maize under drought. Plant Cell Environ. 31, 325-340. [ Links ]
Ashraf, M. and P.J.C. Harris. 2004. Potential biochemical induction of salinity tolerance in plants. Plant Sci. 166, 3-16. [ Links ]
Bray, E.A., J. Bailey-Serres, and E.Weetilnyk. 2000. Responses to abiotic stresses. pp. 1158-1249. In: Gruissem, W., Buchannan,B. and R. Jones, (eds.). Biochemistry and molecular biology of plants. American Society of Plant Physiologists, Rockville, MD, [ Links ]
Caruso, G., C. Cavaliere, P. Foglia, R. Gubbiotti, C. Guarino, R.Samperi, and A. Laganà. 2009. Analysis of drought responsive proteins in wheat (Triticum sativumdurum) by 2D-PAGE and MALDITOF mass spectrometry. Plant Sci., 177(6), 570-576. [ Links ]
Choi, H., J.H. Hong, J. Ha, J.Y. Kang, and S.Y. Kim. 2000. ABFs, a family of ABA-responsive elements binding factors. J. Biol. Chem. 275, 1723-1730. [ Links ]
FAO. 2011. Crop prospects and food situation. Rome. [ Links ]
FAO. 2009. Crop prospects and food situation. Rome. [ Links ]
FAO. 2006. The state of food insecurity in the world. Rome. [ Links ]
FAO. 2005. Global network on integrated soil management for sustainable use of salt-affected soils. FAO Land and Plant Nutrition Management Service, Rome. [ Links ]
Finnie, C. and B. Svensson. 2009. Barley seed proteomics from spots to structures. J. Proteomics 72, 315-324. [ Links ]
Fujita, M., Y. Fujita, K. Maruyama, M. Seki, K. Hiratsu, M. Ohme- Takagi, L.S.P. Tran, K. Yamaguchi-Shinozaki, and K. Shinozaki. 2004. A dehydration-induced NAC protein, RD26, is involved in a novel ABA-dependent stress-signaling pathway. Plant J. 39, 863-876. [ Links ]
Goday, A. and M. Pagés.2004. Proteínas de respuesta al estrés hídrico. pp. 791-832. In: Reigosa, M.J., N. Pedrol, and A. Sánchez-Moreiras (eds.). La ecofisiología vegetal: una ciencia de síntesis. Paraninfo, Madrid. [ Links ]
Hajheidari, M., A. Eivazi, A.B. Buchanan, J.H. Wong, I. Majidi, and G.H. Salekdeh. 2007. Proteomics uncovers a role for redox in drought tolerance in wheat. J. Proteome Res. 6, 1451-1460. [ Links ]
Hashiguchi, A., N. Ahasan, and S. Komatsu. 2009. Proteomics application of crops in the context of climate changes. Food Res. Intl. 43(7), 1803-1813. [ Links ]
Huo, C.M., B.C. Zhao, R.C. Ge, Y.Z. Shen, and Z.J. Huang. 2004. Proteomic analysis of the salt tolerance mutant of wheat under salt stress. Yi Chuan XueBao 31, 1408-1414. [ Links ]
IPCC, International Panel on Climate Change. 2007. Climate change 2007. In: http://www.ipcc.ch; consulted: June, 2011. [ Links ]
Khan, S.V., L. Hoffmann, J. Renauty, and J.F. Hausman. 2007. Current initiatives in proteomics for the analysis of plant salt tolerance. Curr. Sci. 93(6), 807-817. [ Links ]
Khan, M., J. Jan, H. Karibe, and S. Komatsu. 2005. Identification of phosphoproteins regulated by gibberellins in rice leaf sheath. Plant Mol. Bio. 58, 27-44. [ Links ]
Ke, Y., G. Han, H. He, and J. Li. 2009. Differential regulation of proteins and phosphoproteins in rice under drought stress. Biochem. Biophys. Res. Comm. 379, 133-138 [ Links ]
Kreps, J.A., Y. Wu, H.S. Chang, T. Zhu, X. Wang, and J. Harper. 2002. Transcriptoma changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol. 130, 2129-2141. [ Links ]
Komatsu, S. and N. Ahsan. 2009. Soybean proteomics and its application to functional analysis. J. Proteomics 72, 325-336. [ Links ]
Kundzewicz, Z., U. Ulbrich, T. Brücher, D. Graczyk, A. Krüger, and G. Leckebusch. 2005. Summer floods in central Europe – Climate change track? Nat. Hazards. 36, 165-189. [ Links ]
Leshem, Y., Y. Kuiper, P.J.C. Erdei, S. Lurie, and R. Peri-Treves. 1998. D Selye's mammalian "GAS" concept and "co-stress" response exist in plants? pp. 199-208. In: Csermely, P. (ed.). Stress of life: form molecules to man. Vol. 851. New York of Academy of Sciences,. New York, NY. [ Links ]
Lichtenthaler, H.K. and C. Schindler. 1992. Studies on the photoprotective function of zeaxanthin at high-light conditions. pp. 517-520. In: Murata, N. (ed.). Research photosynthesis. Vol. 4. Kluwer Academic Publisher, Dordrecht, The Netherlands. [ Links ]
Mozarfar, A. and J.J. Oertly. 1990. Multiples stress and growth of barley: Effect of salinity and temperature shock. Plant Soil 128, 153-160. [ Links ]
Munns, R. 2005. Genes and salt tolerance: Bringing them together. New Phytol. 167, 645-663. [ Links ]
Ndimba, B.K., S. Chivasa, W.J. Simony, and A.R. Slabas. 2005. Identification of Arabidopsis salt and osmotic stress responsive proteins using two dimensional difference gel electrophoresis and mass spectrophotometry. Proteomics 5, 4185-4196. [ Links ]
Prasad, M.N.V. and Z. Rengel. 1998. Plant acclimation and adaptation to natural and antropogenic stress. pp. 216-223. In: Sermely, P.C. (ed.). Stress of life: form molecules to man. New York of Academy of Sciences. New York, NY. [ Links ]
Qureshi, M.I., S. Qadir, and L. Zolla. 2007. Proteomics-based dissection of stress-responsive pathways in plants. J. Plant Physiol. 164, 1239-60. [ Links ]
Qureshi, M.I., M. Israr, M.Z. Abdin, and M. Iqbal. 2005. Responses of Artemisia annual L. to lead and salt-induced oxidative stress. Environ. Exp. Bot. 53, 185-195. [ Links ]
Rabbani, M.A., K. Maruyama, H. Abe, M.A. Khan, K. Katsura, Y. Ito, K. Yoshiwara, M. Seki, K. Shinozaki, and K. Yamaguchi- Shinozaki. 2003. Monitoring expression profiles of rice (Oryza sativa L.) genes under cold, drought and high-salinity stresses, and ABA application using both cDNA microarray and RNA gel blot analyses. Plant Physiol. 133, 1755-1767. [ Links ]
Rabello, A.R., C.M. Guimarães, P.H. Rangel, F.R. da Silva, D. Seixas, and E. de Souza. 2008. Identification of drought-responsive genes in roots of upland rice (Oryza sativa L.). BMC Genomics 9, 485-497. [ Links ]
Ribas-Carbo, M., N.L. Taylor, L. Giles, S. Busquets, P.M. Finnegan, and D.A. Day. 2005. Effects of water stress on respiration in soybean leaves. Plant Physiol. 139, 466-473. [ Links ]
Sakuma, Y., K. Maruyama, Y. Osakabe, F. Qin, M. Seki, K. Shinozaki, and K. Yamaguchi-Shinozaki. 2006. Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought responsive gene expression. The Plant Cell 18, 1292-1309. [ Links ]
Schwieger, J., M. Lang, and H.K. Lichtenthaler. 1996. Differences in fluorescence excitation spectra of the leaves between stressed and non-stressed plants. J. Plant Physiol. 148, 536-547. [ Links ]
Shinozaki, K. and K. Yamaguchi-Shinozaki. 2007. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 58(2), 221-227. [ Links ]
Shinozaki, K., K. Yamaguchi-Shinozaki, and M. Seki. 2003. Regulatory network of gene expression in the drought and cold stress responses. Curr. Opin. Plant Biol. 6, 410-417. [ Links ]
Timperio, A.M., M.G. Egidi, and L. Zolla. 2008. Proteomic applied on plant abiotic stresses: role of the heat shock proteins (HSP). J. Proteomics 71, 391-411. [ Links ]
Tobari, S., M. Wissuwa, M. Heidari, and M.R. Naghavi. 2009. A comparative proteome approach to decipher the mechanism of rice adaptation to phosphorous deficiency. Proteomics 9, 159-170. [ Links ]
Umezawa, T., M. Fujita, Y. Fujita, K. Yamaguchi-Shinozaki, and K. Shinozaki. 2006. Engineering drought tolerance in plants: discovering and tailoring genes unlock the future. Curr. Opin. Biotechnol. 17, 113-122. [ Links ]
Uno, Y., T. Furihata, H. Abe, R. Yoshida, K. Shinozaki, and K. Yamaguchi-Shinozaki. 2000. Arabidopsis basic leucine zipper transcriptional transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proc. Natl Acad. Sci. USA 97, 11632-11637. [ Links ]
Veeranagamallaiah, G., G. Jyothsnakumari, M. Thippeswamy, P. Chandra Obul Reddy, G. Sriranganayakulu, Y. Mahesh, B. Rajasekhar, Ch. Madhurarekha, and C. Sudhakar. 2008. Proteomic analysis of salt stress responses in foxtail millet (Setaria italica L. cv. Prasad) seedlings. Plant Sci. 175, 631-641. [ Links ]
WWF, World Wild Foundation. 2008. The 2008 living planet report. Switzerland. [ Links ]
Yan, S., Z. Tang, W. Su, and W. Sun. 2005. Proteomic analysis of salt stress-responsive proteins in rice roots. Proteomics 5, 235-244. [ Links ]
Yoshimura, K., A. Masuda, M. Kuwano, K. Yokota, and K. Akashi. 2008. Programmed proteome response for drought avoidance/ tolerance in the root of a C(3) xerophyte (wild watermelon) under water deficits. Plant and Cell Physiol., 49, 226–241. [ Links ]
Zeidler, J.G. and H.K. Lichtenthaler. 2001. Biosynthesis of 2-metyl- 3-buten-2-ol- emitted from needles of Pinus ponderosa via the non-mevalonato DOXP/MEP pathway of isoprenoid formation. Planta 231, 323-326. [ Links ]