Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Agronomía Colombiana
Print version ISSN 0120-9965
Agron. colomb. vol.29 no.2 Bogotá June/Aug. 2011
SOILS, PLANT NUTRITION & WATER MANAGEMENT
Microbial activity in soil and sediments of the upper Arzobispo River basin
Actividad microbiana en suelos y sedimentos de la cuenca alta del Río Arzobispo
Laura Emilia Cerón R.1, 2 and Eduardo Ramírez V.1
1Department of Basic Science, Universidad EAN. Bogota (Colombia).2Corresponding author. leceron@unal.edu.co Recceived for publication: 22 January, 2010. Accepted for publication: 2 June, 2011.
ABSTRACT
Soil biochemical properties are indicators of its quality, properties related to biological cycles of the elements. The enzymatic activities play a key role in the biochemical functioning of soils, including the formation and degradation of organic matter, nutrient cycling and decomposition of xenobiotics. To understand the activity of microbial communities and their effect on the dynamics of organic soil matter, it is necessary to focus on two aspects of the dynamics of the biota and the matrix. This study consisted of an approach to identify the measures that cover the state of health and quality of soils and sediments: the enzymatic activities: acid phosphatase, alkaline phosphatase, dehydrogenase and o-diphenol oxidase. It can be seen that the response of the evaluated activities and their possible relationship to the gradient of pollution in the river upstream of Arzobispo indicate a threat of degradation.
Key words: gradient of pollution, acid phosphatase, alkaline phosphatase, dehyddrogenase, and o-diphenol oxidase.
RESUMEN
Las propiedades bioquímicas del suelo son indicadores de su calidad, propiedades relacionadas con los ciclos biológicos de los elementos. Las actividades enzimáticas desempeñan un papel clave en el funcionamiento bioquímico de los suelos, incluidos los de formación y degradación de la materia orgánica, reciclaje de nutrientes y la descomposición de los xenobióticos. Para entender la actividad de las comunidades microbianas y su efecto sobre la dinámica materia orgánica de suelo, es necesario enfocarse en dos aspectos la dinámica de la biota y de la matriz. Este trabajo consistió en un acercamiento para identificar las medidas acerca del estado de salud y calidad de suelos y sedimentos: las actividades enzimáticas: fosfatasa acida, fosfatasa alcalina, deshidrogenasa y o-difenol oxidasa. Se puede considerar que la respuesta de las actividades evaluadas y su posible relación con el gradiente de contaminación presente en la cuenca alta de rio Arzobispo, indican amenaza de degradación de los suelos.
Palabras clave: gradiente de contaminación, fosfatasa ácida, fosfatasa alcalina, deshidrogenasa, o-difenol oxidasa.
Introduction
Soil is a vital resource whose operation depends on the condition and ability to function as a filter, absorbing and transforming material to protect the environment and water sources from pollution (Doran, 2002; Doran and Zeiss, 2000) among other functions, including microbial metabolic processes involved in the decomposition of organic materials and detoxification of xenobiotics, processes where catalysis of soil has a major role. To get closer to understanding the levels of microbial activity responsible for the processes involved in bioremediation and degradation of xenobiotics and pollutants and the status of nutrient cycling during the development of such processes enzymatic activities in the soil were studied because they relate to: soil biology, since their presence is directly dependent on continued release into the environment carried out by living organisms in the ecosystem (Burns, 1982), and are related to ecological functions such as biomass production, remediation of contaminants and conservation of ecosystems. In addition, they are rapid and sensitive indicators to measure the level of soil degradation (Cerón and Melgarejo, 2005) in natural and agricultural ecosystems, and therefore are suitable for measuring the impact of pollution on the quality of this resource.
Anthropogenic factors have taken the lead role in the changes that ecosystems may suffer so it is necessary to define how and at what intensity they are affected. The main causes of degradation and reduced soil productivity are directly related to improper management and intensive cultivation without rotation and accidental or deliberate contamination with domestic and industrial wastes (Gianfreda et al., 2005). To determine the impact of contaminants and potential remedial ecosystem measures needed related to the metabolic status of the community, since the influence of contaminants on microbial communities, as well as the ability to use them as source nutrients,are related and dependent on other soil properties.
River ecosystems around the world are impacted by activities such as agriculture, urbanization, domestic and industrial effluents and channel modification, resulting in alteration and destruction. In Bogota, the rivers have been used as collectors of both wastewater and storm water in the city, whose highly concentrated flows greatly degrade the environment and water quality. To protect works civil from bordering rivers, sometimes gabion walls or dikes are constructed as corrective measures (Jarillones) to avoid overflow, but these works reduce the cross sections, resulting in increases in flow velocity and water level rise.
The Arzobispo River is a principal environmental structure, as it is the main source of the river Salitre or Juan Amarillo one of the three main drains of the city, originating in the eastern hills and entering the city limits between Avenida Circunvalar and Avenida Carrera 7, recovering and maintaining its abundance of water and biodiversity, means improving the quality of the water and reducing pollution from the Bogotá River. The main sources of water pollution in this river come from the sewer system (Olaya-Álvarez, 2010): domestic sewage, industrial discharges and illegal connections to the sewer system, rain flow, bringing an increase of matter organic, pathogens, heavy metals and other pollutants. Recently attention has been paid to the preservation of flood plains along rivers; given their importance in improving water quality and the biodiversity they support (Subrahmanyan et al., 2011; Kang and Stanley, 2005). In addition, few studies have been done on the canalization of rivers and wetlands and their impact on ecosystem processes and degradation of natural resources.
Biochemical indicators are inherently more sensitive to environmental conditions than mineral properties which can be used to assess the state of soil degradation because they are sensitive to environmental stress conditions (Chaer et al., 2009), and so provide information about the interactions between natural biochemical processes and the effects of anthropogenic activities. The variation in dehydrogenase activity reflects the status of degradation / rehabilitation of soils (Doi and Ranamukhaarachchi, 2009) and is considered as a measure of microbial activity that responds to environmental gradients an integrated measure of soil quality, so it is widely used (Maliszewska-Kordybuch and Smreczek, 2003) in soil and ecotoxicological tests in aquatic ecosystems (Weaver et al., 2011; Hill et al., 2010; Hill et al., 2006). The phosphatase activity catalyzes the hydrolysis of phosphate esters, released phosphate groups bound to be in more complex substrates such as soil organic matter, a relationship between this activity, the quality and quantity of organic matter and total phosphorus content has been found in aquatic ecosystems (Wang and Panta, 2010; Cunqi et al., 2007; Wilczek et al., 2005) as well as (Cunqi et al., 2007) a mechanism of substrate induction and inhibition by product. The o-diphenol oxidase activity catalyzes oxidation of phenol compounds, the importance of phenol acids and microbiological released during hydrolysis of naturally occurring substances (plant residues and organic) in soils and synthetic compounds (waste industrial and pesticides) has been demonstrated in the formation of humic substances (Perucci et al., 2000). Phenol products are generally unstable and are subject to oxidative conversion by abiotic reactions or catalyzed by oxidative enzymes to produce quinones which polymerize to humus-like macromolecules in the presence or absence of amino compounds. Phenol compounds in soil are mainly transformed by oxidative processes catalyzed by phenolase and peroxidase produced by the microbiota present in soil.
The aim of this study was to evaluate the effect of the pollution gradient on microbial activity present in sediments and soils adjacent to the upper basin of the Arzobispo River and its relationship with the quality of water resources.
Method and materials
Study site and sampling
Soil and sediment samples were taken surrounding the upper reaches of the Arzobispo River, that is from the point of entry to the city limits in Olaya Herrera National Park to Carrera 7ª, 250 m running along the river where it is partially channeled. From the Convention 005 of 2006 made between the Secretaría Distrital de Ambiente (SDA) and the Empresa de Acueducto y Alcantarillado de Bogota (EAAB), the first stretch of the river was named where the water quality ranges from questionable to acceptable (Olaya-Álvarez, 2010; EAAB, 2007) generating a pollution gradient which results in the detriment of quality of water resources, as evaluated from water quality parameters reported by the SDA and EAAB (Tab.1), associated with a progressive increase in pH of the water and soils that are found in the present study, the discharges that contribute organic matter, total suspended solids and fecal coliform. In this first section of the river, three equidistant places were chosen and named: 1st Avenue Circunvalar, 2nd Coliseum and 3rd Cra 5a, samples were taken during two seasonal periods, the dry period in September and the rainy period in November 2009. The distribution throughout the year is bimodal in both the first half and the second half of the year, the highest rainfall occurs in the second half of October and November, with a rainfall of around 110 mm, the driest months are July to early September with an annual rainfall of approximately 43 mm (Olaya-Álvarez, 2010). Soil samples were taken at a depth of between 5 and 10 cm and surface sediment were taken from 0 to 5 cm, at each sampling site nine samples were taken in a W scheme to form three composite samples, which were then transported in bags and refrigerated at the laboratory. Soils and sediments were passed through a sieve (2 mm), dissected and homogenized and then stored at -20°C. Water content was determined for each of the samples by observing the weight difference before and after drying in an oven at 72°C for 48 h.
Enzyme activities
The enzyme activities evaluated were acid phosphatase (EC 3.1.3.2), alkaline phosphatase (EC 3.1.3.1), dehydrogenase (EC 1.1.1.1) and o-diphenol oxidase (EC 1.10.3.1) in soils and sediments, as they are parameters that relate to ecosystem properties and therefore provide information about the impact of pollution on the resource. Evaluation of acid phosphatase (EC 3.1.3.2) and alkaline (EC 3.1.3.1) phosphatase activities. The enzymatic assay was based on the determination of Ρ-nitrophenol released by the enzyme from Ρ-nitrophenyl phosphate substrate, which was incubated with a soil sample for 1 h at 37°C in a pH 6.5 buffer to determine acid phosphatase and pH 11 to determine alkaline phosphatase . The Ρ-nitrophenol released by activity in alkaline medium x and determined colorimetrically at 400 nm. Dissolved humic substances increase the alkaline medium and can interfere with the determination of Ρ-nitrophenol, which is why calcium chloride is added to the samples to prevent dispersion of clay minerals (Tabatabai, 1982; Eivazi and Tabatabai, 1977). Evaluation of dehydrogenase activity (EC 1.1.1.1). The enzymatic assay was based on the determination of triphenyl formazan formed from to reduction of TTC TPF chloride in the soil, the TCC serves as an electron acceptor for several dehydrogenase s (Casida et al., 1964). The measurement of this enzymatic activity in soil systems comprising different dehydrogenase s and its origin is biological oxidation by dehydrogenation processes of different organic compounds, which under aerobic conditions are linked electron transport chain coupled to the synthesis of ATP, which has oxygen as a final acceptor, so it is considered a measure of microbial activity in situ.
Evaluation of o-diphenol oxidase activity (EC 1.10.3.1). The most important problem in the study of the oxidation of phenol compounds in soils is the low availability of oxidized substrate caused by the sorption of soil components at the interface with enzymatic catalysis because reactions occur immediately after catechol contact with the ground. The quinones formed by oxidation of phenol compounds are highly reactive and able to form various condensation products especially in the presence of compounds with free amino groups, this method is based on the formation of red compounds, through the development of the enzymatic oxidation of catechol and subsequent reaction with proline, using colorimetric determination at 525 nm of red compounds derived from the reaction between products of the enzymatic reaction and proline (Perucci et al., 2000). Evaluation of soil pH, we performed soil suspension in distilled water in a 1:2.5 ratio.
Statistical analysis
To compare the effect of different sampling sites on the enzyme activities we tested for analysis of variance as a completely randomized design (P=0.05), we used multiple comparison tests to determine differences between means, however small the significant difference. Analyses were performed using SPSS 17.0 statistical software.
Results and discussion
As we descended the study area 1st Avenida Circunvalar, 2nd Coliseo and 3rd Cra 5a , we found a progressive increase in pH in the samples of water and surrounding soils (Tab.1) and the presence of contaminants in the water. The increase in pH in the water of the river at Cra 5a is related to the presence of misguided urban wastewater connections which provide organic matter, total suspended solids, nitrogen,phosphorus, pH, total and fecal coliforms, which decrease dissolved oxygen and water quality. This gradient is also influenced by the degree of intervention by construction of dikes.. Urban waste water containing significant levels of nitrogen (ammonium, nitrite nitrate), phosphorus and micronutrients such as Fe, Cu and Zn, which can be removed by irrigation to soil (Brzezinska et al., 2001), as the soil can act as a sort of natural filter when irrigated with wastewater (Chen et al., 2008).
Enzyme activities
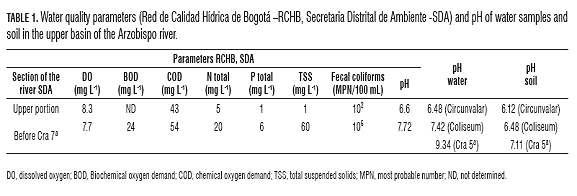
Phosphate activity catalyzes the hydrolysis of phosphate esters, releasing phosphate groups bound in more complex substrates such as organic soil matter, thus is responsible for the mineralization of organic phosphorus in the form of inorganic phosphorus which is available for the requirements of microorganisms and plants. This activity is classified as acidic (pH 6.5) or alkaline (pH 11) according to the optimal pH. The acid phosphatase are derived from both plants and microorganisms, while the alkaline are derived primarily from microorganisms. The results obtained for acid phosphatase activity (mol Ρ-nitrophenol/g dry soil per h) in soils and sediments from the upper basin of the Arzobispo taken in the two seasons, the dry and wet periods (Fig.1).
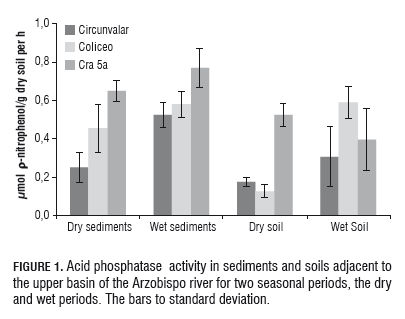
The acid phosphatase activity was significantly higher (P=0.05) in sediments from the Cra 5a site, compared to the same sediments evaluated in Circunvalar and Coliseo, for the two sampling times. The activity was significantly higher in soils from Cra 5a than in soils from Circunvalar and Coliseo in the dry period, while a significant increase was only observed in the activity at Coliseo in the rainy season, compared to what was found for the same point in the dry season. The results obtained for alkaline phosphatase activity (mol of Ρ-nitrophenol/g dry soil per h) in soils and sediments taken in the two seasons, the dry period and the wet period (Fig.2).
The alkaline phosphatase activity was significantly higher (P=0.05) in sediments from the Cra 5a site, in relation to the same sediments evaluated at Circunvalar for the two sampling times. While no differences were observed between Circunvalar and Coliseo for the two seasonal periods in which sampling was performed, significant differences were found in the assessed activity in soils among the three sampling points for the dry period, with the lowest activity found at Coliseo and the highest at Cra 5a. The activity showed a significant recovery with the effect of rainfall at Coliseo, as compared to that found in the same place for the dry period, but no significant effect at the sampling site for alkaline phosphatase activity was observed for the rainy season. It has been reported (Wilczek et al., 2005) that enzyme variation in river sediments due to seasonal variations, particularly the increase in total phosphorus, carbon and x phosphatase activity in surface sediments of rivers, naturally increases with the seasonal dynamics when in autumn plant material degradation as well as flooding predominant. It has also been used as an indicator of bioavailability of phosphorus in aquatic systems (Wang and Panta, 2010).
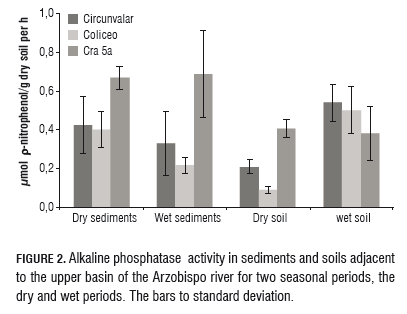
Dehydrogenase activity reflects the work of a group of intracellular enzymes that are present in the soil microbiota, these are part of the metabolic reactions involved in oxidative energy transfer and are considered a good indicator of microbial activity in addition to being sensitive to land degradation, and are also affected by seasonal changes. There is evidence (Doi and Ranamukhaarachchi, 2009) to consider dehydrogenase activity as an indicator of the condition of soil and seasonal changes in rainfall/ drought. The results obtained for this study (mg TPF/g dry soil per h) in soils and sediments taken in the two seasons seasonal, the dry and wet periods (Fig.3).
Dehydrogenase activity was significantly (P=0.05) higher in sediments from the Cra 5a site as compared to the Circunvalar and Coliseo sites, for the two sampling times. A significant effect was found by sampling location and time (rain) in the activity of the sediments at the Cra 5a location, with a significantly higher value found, as compared to those recorded in different locations and sampling periods. In soils, a significant effect on the sampling site during the dry season was found, with the most recorded activity at Cra 5a compared to that of Circunvalar, but there was no difference between that of Circunvalar and Coliseo. No differences were found between the activities of the different soils sampled in the rainy season.
The acid phosphatase activity, alkaline and dehydrogenase , present in the sediments of the upper basin of the Arzobispo respond to a gradient of pollution in the river as observed in the present study, probably in response to increases in nutrients as noted above (Hill et al., 2010; Hill et al., 2006) of other river basins. Increases were observed in the activities in soils and sediments with increased nutrients (carbon and nitrogen) in connection with the addition of organic amendments in agricultural soils (Monkiedjea et al., 2006) and urban and industrial waste. Singh and Agrawal (2008) reported increases in these activities in relation to the presence of organic materials derived from municipal waste, it has been reported (Jezierska-Tys and Frac, 2009; Singh and Agrawal, 2008) that there are positive correlations between dehydrogenase activity, the ammonium (NH4+) concentration and pH in response to the daily application of different doses of sewage sludge rich in carbon and nitrogen, but the activities can be inhibited by applications over long periods and the presence of heavy metals, particularly dehydrogenase activity, alkaline phosphatase and ammonification rate. However, it was reported (Carreira et al., 2008) that there is an inhibition of dehydrogenase and acid phosphatase activities in response to heavy metal pollution and recovery of the same after restoration of vegetation in the river floodplains. On the other hand, the canalization of rivers still brings with changes in water availability for surrounding soils and soil chemistry, as well as changes in the structure of plant communities, changes that led to a significant increase in dehydrogenase activities, ß-glucosidase and phosphatase (Kang and Stanley, 2005), and changes in water regimes influence the vegetation composition and dynamics of organic matter.
One can consider that the activities assessed in this study respond to the gradient of contamination present in the upper Arzobispo River, indicating a threat of degradation. Because on the one hand, the variation of soil dehydrogenase activity reflects the degradation/restoration of the land (Doi and Ranamukhaarachchi, 2009) significantly, given their correlation with decreasing density and increasing field capacity, as a criterion for measuring the status of land degradation and land rehabilitation. dehydrogenase activity is considered as a measure of microbial activity, a superior technique involving the observation threshold (Soares et al., 2006), and responds to environmental gradients, serving as an integrated measure of soil quality.
On the other hand, the importance of the phenols and acid products microbiological released during the hydrolysis of naturally occurring substances (plant residues and organic) in soil and synthetic compounds (pesticides and industrial waste), in forming humic substances (Perucci et al., 2000) has been demonstrated. Phenol products are generally unstable and are subject to oxidative conversion by abiotic reactions or catalyzed by oxidative enzymes to quinones and polymerize to humus-like macromolecules in the presence or absence of amino compounds.
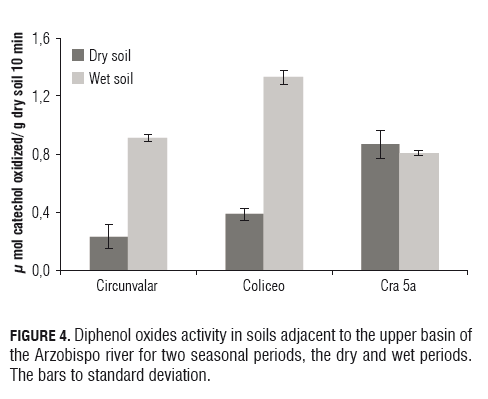
Phenol compounds in soil are mainly transformed by oxidative processes catalyzed by phenolase and peroxidase produced by the microbiota present in the soil. The results obtained for o-diphenol oxidase activity (mol catechol oxidized/g dry soil per h) present in soils taken in the two seasons, the dry and wet periods (Fig.4). The rainy season showed a significant (P=0.05) increase in activity at Circunvalar and Coliseo, but this effect was not found in the soils at Cra 5a. In the present study, the increase of dehydrogenase activity found in soils and sediments at Cra 5a and decreased o-diphenol oxidase activity at the same place may be related to decreased water quality (Tab.1 ) and/or pollution of urban effluents. The o-diphenol oxidase activity is related to the degradation of vegetative material and is directly influenced by the type of vegetation and its expression is linked to the availability of nutrients, it has been observed (Monkiedje et al., 2006) that increased nitrogen and phosphorus nutrients in the soil, have negative effects on the activity of phenol oxides. Just as the introduction of these nutrients in natural aquatic ecosystems have negative influences on the activity (Penton and Newman, 2008), accompanied by decreased plant diversity. It is postulated that increasing plant community diversity can contribute to the enrichment of soil fertility and ecological structure (Beare et al., 1995).
In aquatic ecosystems the detritus food chain is a very important link, where microbial assimilation plays a fundamental role. To find the link between microbial variables and ecosystem processes it is necessary to understand the factors controlling the distribution of microbial biomass and activity involved in the acquisition of carbon and nutrients, which may contribute to a better understanding of the ecosystem-level interactions between nutrients and organic matter and microbial processes and mediate the flow of energy and nutrient recycling.
Enzymes are a measure of the availability of nutrients, so it has been proposed (Hill et al., 2010; Hill et al., 2006) that together with the chemical properties of water quality such as measures of the resource quality, measures of the microbial activity in sediments of rivers, integrated ecosystem conditions linking the concentration of nutrients in the water and the concentration of nutrients in the sediments.
Conclusions
The acid phosphatase activity, alkaline and dehydrogenase, present in sediments was observed to respond to the gradient of pollution in the Arzobispo river, probably in response to increases in nutrients and vegetation change. It can be seen that the response of the activities evaluated in this study indicate a threat of degradation which requires a restoration of environmental management practices to mitigate pollution where such activities can be used as indicators of resource recovery.
Acknowledgements
The authors express their gratitude to the Vice-Rector of Research at the Universidad EAN for funding this study.
Cited literature
Beare, M.H., D.C. Coleman, D.A. Jr. Crossley, P.F. Hendrix, and E.P. Odum. 1995. A hierarchical approach to evaluating the significance of soil biodivesity to biogeochemical cycling. Plant and Soil 170, 5-22. [ Links ]
Brzezinska, M., Z. Stepniewska, and W. Stepniewski. 2001. Dehydrogenase and catalase activity of soil irrigated with municipal wastewater. Pol. J. Environ. Stud. 10, 307-311. [ Links ]
Burns, R.G. 1982. Enzyme activity in soil: location and a possible role in microbial ecology. Soil Biol. Biochem. 14, 423-427. [ Links ]
Carreira, J.A., B. Viñegla, R. García-Ruiz, V. Ochoa, and M.B. Hinojosa. 2008. Recovery of biochemical functionality in polluted flood-plain soils: The role of microhabitat differentiation through revegetation and rehabilitation of the river dynamics. Soil Biol. Biochem. 40, 2088-2097. [ Links ]
Casida, L.E. Jr., D.A. Klein, and T. Santoro. 1964. Soil dehydrogenase activity. Soil Sci. 98, 371-376. [ Links ]
Chaer G.M., D.D. Myrold, and P.J. Bottomley. 2009. A soil quality index based on the equilibrium between soil organic matter and biochemical properties of undisturbed coniferous forest soils of the Pacific Northwest. Soil Biol. Biochem. 41, 822-830. [ Links ]
Cerón R., L.E. and L.M. Melgarejo. 2005. Enzimas de suelo: indicadores de salud y calidad. Acta Biol. Colomb. 10, 5-17. [ Links ]
Chen, W., L. Wu, W.T. Jr. Frankenberger, and A.C. Chang. 2008. Soil enzyme activities of long-term reclaimed wastewater-irrigated soils. J. Environ. Qual. 37(Supp. 5), 36-42. [ Links ]
Cunqi, L., L. Jianjian, and L. Hepeng. 2007. Landward changes of soil enzyme activities in a tidal flat wetland of the Yangtze River Estuary and correlations with physico-chemical factors. Acta Ecol. Sinica 27, 3663-3669. [ Links ]
Doi, R. and S.L. Ranamukhaarachchi. 2009. Soil dehydrogenase in a land degradation-rehabilitation gradient: observations from a savanna site with a wet/dry seasonal cycle. Rev. Biol. Trop. 57, 223-234. [ Links ]
Doran J.W. and M.R. Zeiss. 2000. Soil health and sustaninability: managing the biotic component of soil quality. Appl. Soil Ecol. 15, 3-11. [ Links ]
Doran, J.W. 2002. Soil health and global sustainability translating science into practice. Agric. Ecosyst. Environ. 88, 119-127. [ Links ]
EAAB, Empresa de Acueducto y Alcantarillado de Bogota. 2007. Plan de saneamiento y manejo de vertimientos (PSMV). Bogota. [ Links ]
Eivazi, F. and M.A. Tabatabai. 1977. Phosphatases in soils. Soil Biol. Biochem. 9, 167-172. [ Links ]
Gianfreda, L., M.A. Raoa, A. Piotrowska, G. Palumbob, and C. Colombob. 2005. Soil enzyme activities as affected by anthropogenic alterations: intensive agricultural practices and organic pollution. Sci. Total Environ. 341, 265-279. [ Links ]
Hill, B.H., C.M. Elonen, T.M. Jicha, A.M. Cotter, A.S. Trebitz, and N.P. Danz. 2006. Sediment microbial enzyme activity as an indicator of nutrient limitation in Great Lakes coastal wetlands. Freshw. Biol. 51, 1670-1683. [ Links ]
Hill, B.H., C.M. Elonen, T.M. Jicha, D.W. Bolgrien, and M.F. Moffett. 2010. Sediment microbial enzyme activity as an indicator of nutrient limitation in the great rivers of the Upper Mississippi River basin. Biogeochem. 97, 195-209. [ Links ]
Jezierska-Tys, S. and M. Frac. 2009. Impact of dairy sewage sludge on enzymatic activity and inorganic nitrogen concentrations in the soils. Int. Agrophys. 23, 31-37. [ Links ]
Kang, H. and E.H. Stanley. 2005. Effects of levees on soil microbial activity in a large river floodplain. River Res. Appl. 21(1), 19-25. [ Links ]
Maliszewska-Kordybuch, B. and B. Smreczek. 2003. Habitat function of agricultural soils as affected by heavy metals and polycyclic aromatic hydrocarbons contamination. Environ. Int. 28, 719-28. [ Links ]
Monkiedjea, A., M. Spitellerb, D. Fotioc, and P. Sukulb. 2006. The effect of land use on soil health indicators in peri-urban agriculture in the humid forest zone of southern Cameroon. J. Environ. Qual. 35, 2402-2409. [ Links ]
Olaya-Álvarez, A.M. 2010. Formulación del plan de ordenación y manejo de la cuenca del río Salitre en el perímetro urbano del Distrito Capital. Secretaría Distrital de Ambiente (SDA); Universidad Militar Nueva Granada, Bogota. [ Links ]
Penton, C.R. and S. Newman. 2008. Enzyme-based resource allocated decomposition and landscape heterogeneity in the Florida everglades. J. Environ. Qual. 37, 972-976. [ Links ]
Perucci, P., C. Casucci, and S. Dumontet. 2000. An improved method to evaluate the o-diphenol oxidase activity of soil. Soil Biol. Biochem. 32(1), 387-399. [ Links ]
Singh, R.P. and M. Agrawal. 2008. Potential benefits and risks of land application of sewage sludge. Waste Mgmt. 28, 347-358. [ Links ]
Soares, R.A., L.F.W. Roesch, G. Zanatta, F.A. Camargo, and L.M.P. Passaglia. 2006. Occurrence and distribution of nitrogen fixing bacterial community associated with oat (Avena sativa) assessed by molecular and microbiological techniques. Appl. Soil Ecol. 33, 221-234. [ Links ]
Subrahmanyam, G., G. Archana, and L.S. Chamyal. 2011. Soil Microbial Activity and its relation to soil indigenous properties in semi-arid alluvila and estuarine soils of mahi river basin, Western India. Int. J. Soil Sci. 6, 224-237. [ Links ]
Tabatabai, M.A. and J.M. Bremner. 1969. Use of p-nitrophenyl phosphate for assay of soil phosphataseactivity. Soil Biol. Biochem. 1, 301-307. [ Links ]
Wang, J. and H.K. Panta. 2010. Enzymatic hydrolysis of organic phosphorus in river bed sediments. Ecol. Eng. 36, 963-968. [ Links ]
Weaver, M.A., R. Zablotowicz, K. Larry, L. Martin, and B. Charles. 2011. Microbial and vegetative changes associated with de [ Links ]