Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Agronomía Colombiana
Print version ISSN 0120-9965
Agron. colomb. vol.29 no.3 Bogotá Sept./Dec. 2011
SOILS, FERTILIZATION AND MANAGEMENT OF WATER
Silicon and plant diseases. A review
El silicio y las enfermedades de las plantas. Una revisión
Alicia Romero1,4, Fernando Munévar2, and Gerardo Cayón3
1Department of Chemistry, Faculty of Sciences, Universidad Nacional de Colombia. Bogota (Colombia).2External consultant, Bogota (Colombia).
3Department of Agronomy, Faculty of Agronomy, Universidad Nacional de Colombia. Bogota (Colombia).
4Corresponding author. aaromerof@unal.edu.co Received for publication: 11 November, 2010. Accepted for publication: 2 November, 2011.
ABSTRACT
Disease is one of the main limitations on the amount and quality of crop production, by reducing the availability, absorption, distribution and use of nutrients by the plant. Silicon (Si) is one of the most abundant elements in the lithosphere and most soils have considerable amounts. Although not considered an essential nutrient for most plants, a lot of evidence shows the beneficial effects of nutrition with Si on growth, development and health of crops. Many studies have suggested that Si activates the defense mechanisms of plants, but the exact nature of the interaction between this element and the biochemical pathways that direct resistance still remains unclear. This article presents a review of the relationship between mineral nutrition and disease development and discusses the beneficial effects of silicon in crops, its mobility in soil, the process of assimilation through the roots and its influence on tolerance to fungal diseases.
Key words: mineral nutrition, plant diseases, beneficial elements.
RESUMEN
Las enfermedades constituyen una de las principales limitaciones de la cantidad y calidad de la producción de los cultivos porque reducen la disponibilidad de los nutrientes, su absorción, distribución y utilización por la planta. El silicio (Si) es uno de los elementos más abundantes en la litosfera y la mayoría de los suelos presentan cantidades considerables de este elemento. Aunque el Si no se considera un nutriente esencial para la mayoría de las plantas, muchas evidencias demuestran los efectos benéficos de la nutrición con Si sobre el crecimiento, desarrollo y estado sanitario de los cultivos. Muchos estudios han sugerido que el Si activa los mecanismos de defensa de la planta, pero la naturaleza exacta de la interacción entre este elemento y las vías bioquímicas que dirigen la resistencia permanece aún sin esclarecer. En este artículo se presenta una revisión sobre las relaciones entre la nutrición mineral y el desarrollo de las enfermedades y se discuten los efectos benéficos del silicio en los cultivos, su movilidad en el suelo, el proceso de asimilación a través de las raíces y su influencia en la tolerancia a enfermedades causadas por hongos.
Palabras clave: nutrición mineral, enfermedades de plantas, elementos benéficos.
Introduction
Mineral nutrition influences the growth and production of crops and causes changes in the pattern of growth, morphology and anatomy, and particularly the chemical composition of the plants. It has been established that mineral nutrients may increase or decrease the tolerance of plants to pathogens and pests, and are considered important factors in controlling diseases (Huber and Graham, 1999; Marschner, 2002) and form part of the chemical environment of the soil-plant system, and their management is of potential usefulness in plant control (Munévar, 2004). In fact, the severity of the majority of plant diseases can be reduced by improved mineral nutrition management. This can be achieved by modifying the availability of particular nutrients or improving the efficiency of absorption and utilization by the plant (Huber, 1997; Hodson et al., 2005). The supply of nutrients through fertilization or modification of the soil environment influences the availability of nutrients, and constitutes a form of plant disease control and is an integral component of agricultural production (Huber and Graham, 1999). Many farming practices such as crop rotation, application of organic amendments, adjusting the pH of soil, weed control and maintenance of irrigation often influence disease through interactions with mineral nutrients. These practices directly supply nutrients or increase availability to plants through the alteration of soil biological activity (Huber, 1997; Solomon et al., 2003; Turner, 2003; Hodson et al., 2005; Huber and Haneklaus, 2007). However, the mechanisms by which nutrition induces changes in the development of diseases are complex and diverse and include effects of mineral nutrients directly on pathogens and development of the plant and its mechanisms of resistance (Munévar, 2004; Walters and Bingham, 2007).
Interactions between nutrition and plant diseases are very complex with dynamics and results that depend on many factors including plant species, growth stage and biotic and abiotic factors. The level of severity of most diseases can be reduced through proper management of nutrients. This article analyzes and discusses the latest information on the relationship of silicon with some plant diseases.
Silicon (Si)
According to the classical definition of essentiality (Arnon and Stout, 1939), Si has not been identified as an essential nutrient for plants, but some authors (Epstein, 1994; Ma and Takahashi, 2002; Ma and Yamaji, 2008; Datnofft et al., 2007; Pilon-Smits et al., 2009) have extensively documented the beneficial or favorable effects of Si nutrition on growth, development and health status of plants. Si has been identified as a bioactive and beneficial element in some species (Richmond and Sussman, 2003) to the point that its potential benefits on growth and behavior of some species have been extensively reviewed (Epstein, 1994). These favorable effects include increased growth and production, improvements in some morphological characteristics (height, root penetration into the soil, exposure of leaves to light, resistance to lodging), reduced transpiration and resistance to stress, resistance to salinity and toxic metal toxicity, effects on enzyme activity and increased resistance to pathogens. Although some of these properties are probably derived from the setting of amorphous silica deposits (SiO2·nH<sub>2</sub>O), others may be considered as a consequence of the bioactivity of monosilicic acid (Fauteux et al., 2005). The positive effects produced by Si in plants have been attributed to: 1) reduction of water loss by cuticular transpiration caused by the formation of deposits of Si beneath the cuticle, 2) decreased apoplastic flow and reduced absorption of toxic minerals due to the formation of deposits of Si on the root, and 3) increased stiffness and strength of plant cell wall (Ma and Yamaji, 2006; Ma and Yamaji, 2008). Below are some general aspects of Si in soil and plants, their effect on disease control in some crops and mechanisms of action through which the resistance of plants is possibly mediated by this element.
Si in the soil
Si is the second most abundant element in soil and is approximately 28% of the Earth's crust. Si available in the soil for plants is in the form of monosilicic acid, Si(OH)4 (Sommer et al., 2006; Currie and Perry, 2007). Monosilicic acid is found in soils in concentrations between 0.1 and 0.6 mM (McKeague and Cline, 1963; Savant et al., 1997a; Savant et al., 1997b) as monosilicic acid [Si(OH)4] or its ionized form, Si(OH)3O-, which predominates at pH values greater than 9.0. Si is also found in silicate minerals and may be adsorbed or precipitated with oxides of Al, Fe and Mn. Monosilicic acid concentration in the soil solution, available to the roots, is affected by its dissolution from soil minerals (crystalline and amorphous), adsorption on or desorption from the oxides and hydroxides of Al, Mn, Fe and its dissociation from the polymer of Si(OH)4 (McKeague and Cline, 1963).
Some plants, like microorganisms, have mechanisms to remove the insoluble forms of Si from the ground, like producing acidity on the surface of their roots or chelating agents to release rhizosphere, processes that increase the concentration of monosilicic acid in the soil solution (Winslow, 1995). McKeague and Cline (1963) reported that the concentration of Si in the soil solution is controlled by a pH dependent reaction by which the sesquioxides, especially aluminum oxide, adsorbed monosilicic acid. Aluminum sesquioxide is recognized as adsorbing monosilicic acid in the soil at levels which can increase with increasing pH (Jones and Handreck, 1967). The concentration of sesquioxides of Fe and Al in soil is positively correlated with the adsorption of Si. There is also evidence that the Si can be released from the complex Fe-Si present in the soil (Jones and Handreck, 1967). Reifenberg and Buckwold (1954) showed that phosphorus (P) on the ground, as orthophosphates, affects the release of Si in the soil solution, thus increasing the amount of kaolinite and phosphate added to seven different types of soil obtained large amounts of Si in the soil extracts. The authors suggested that Si and P compete for binding sites on clay and, as P is absorbed, Si is released into the solution.
Despite the abundance of soluble Si in most of the world's mineral soils, its deficiency may occur as a consequence of depletion of this element due to continuous cultivation of crops which require high amounts of Si, as in the case of rice. This crop can absorb between 230 and 470 kg ha-1 of Si and, given the intensity with which it is grown, Si is removed from the soil more rapidly than can be naturally replaced (Savant et al., 1997a; Savant et al., 1997b). Si deficiency occurs most often in Oxisols and Ultisols, which are cultivated with rice in Asia, Africa and Latin America. In regions with high rainfall, where these two soil types occur, different processes can occur such as filtration and desilification (Savant et al., 1997a; Savant et al., 1997b; Datnofft et al., 2007). Histosols also have lower amounts of Si available to plants due to its high content of organic matter (80%) and low mineral content, while Entisols has a high content of quartz (SiO2) in the sand, but the Si is just slightly soluble and unavailable to plants (Datnofft et al., 2007).
Si in plants
Like many macronutrients, the concentration of Si in plants varies between 0.1 and 10% by weight on a dry basis. Plants that have lower Si contents are structurally weaker and more prone to abnormal growth, development and reproduction (Epstein, 1994; Epstein, 1999). The presence of Si in the plant is the result of its absorption from the soil in a soluble form such as monosilicic acid, also called orthosilicic acid Si(OH)4, and its controlled polymerization in a final location, but the ability of the plants to accumulate Si varies widely between species. A high accumulation of Si in monocots has been determined, as well as that the different parts of the same plant can have large differences in the accumulation of Si (Currie and Perry, 2007). Si absorbed by diffusion and absorption of the roots is induced by perspiration by the mass flow process. The species of the family
Poaceae (grasses) accumulate Si at levels commensurate with their rate of transpiration (Jones and Handreck, 1967). When Si is absorbed by the plant in the form of phytoliths or silica bodies (SiO2·nH2O) which occupy spaces between the root (apoplast of the cortex) and the cell walls of some of the cells of the plant, for example those of leaves (Yoshida et al., 1962a; Prychid et al., 2004; Currie and Perry, 2007; Datnofft et al., 2007), it accumulates in a higher amount in mature leaves than in young ones (Ma et al., 1989; Ma and Takahashi, 2002). Some studies indicate that in rice, most of the accumulated Si deposits in the leaves (Chen, 1990; Epstein, 1999) and once it is deposited, it is immobilized and is not redistributed to the growing tissues due to low mobility in the phloem (Datnofft et al., 2007).
The silica particles grow to a size of about 1 to 3 nm and are negatively charged such that they can interact with the local environment of the cell walls of plants. It has been suggested that the nucleation and growth of these structures are under the control of specific proteins (Harrison, 1996; Perry and Tucker, 2000) and that a fraction of Si form bonds with proteins, phenolic compounds (lignin, condensed polyphenols) , lipids and polysaccharides (cellulose) (Kolesnikov and Gins, 2001). Although it has been established that Si interacts with cell wall components (Pilon-Smiths et al., 2009), the nature of this association is not yet completely understood (Perry and Lu, 1992; Currie and Perry, 2007). It has been found that several factors affect the condensation processes of silica, among which are included silicic acid concentration, temperature, pH and the presence of other ions, small molecules and polymers (Fauteux et al., 2005; Currie and Perry, 2007).
Plants are considered accumulators of Si at concentrations greater than 1% of dry weight (Epstein, 1999). Dicots, such as tomatoes and soybeans, with a percentage less than 0.1% in their biomass accumulate less Si compared to the grass monocots such as corn, oats, rye and wheat, which contain about 1% of Si in their biomass, while some aquatic species have contents exceeding 5% (Jones and Handreck, 1967; Epstein, 1999; Datnofft et al., 2007). Plant species belonging to the families Poaceae and Cyperaceae absorb Si at concentration levels equal to or greater than some of the essential nutrients like N and K (Savant et al., 1997b). The Si/Ca ratio is another criterion used to determine whether a plant species is classified as a Si accumulator (Takahashi et al., 1990; Datnofft et al., 2007).
Si in controlling fungal diseases
Probably the first researcher to suggest that Si was involved in rice's resistance to attack by the fungus Magnaporthe grisea was Onodera (1917), who showed the results of a comparative study of the chemical composition of rice plants from 13 different regions in western Japan, where infected plants always had lower concentrations of Si than healthy ones despite having grown under the same conditions. These results do not necessarily indicate that the incidence of disease was reduced by the concentration of Si or plants with lower concentrations were more susceptible but showed that there could be a relationship between concentrations of Si and the susceptibility of the rice plant to disease. This study began a series of investigations into the possible relationship between Si and diseases of rice in Japan. Then, Kawashima (1927) showed that, under controlled conditions, the application of Si to rice plants increased resistance to attack by the fungus M. grisea and that this increase in resistance was higher as the concentration of Si applied in the soil increased.
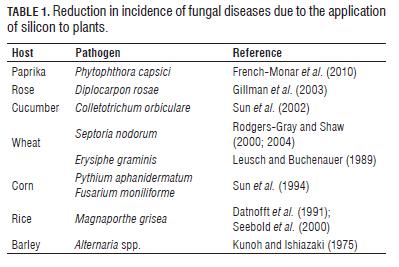
The effects of Si on the reduction in the incidence and severity of plant diseases have been widely reported (Fauteux et al., 2005). The favorable effect of Si in the control of fungal diseases of monocots, mainly rice and other grasses, has been documented since the 60s (Jones and Handreck, 1967). The way in which Si is able to exert its protective effect has not yet been fully understood and so far, functions including physical and biochemical protection systems have been proposed (Currie and Perry, 2007). Tab.1 shows some references which reported a decreased incidence of certain diseases caused by fungus, due to the application of silicon.
Mechanical protection of plants caused by Si A notable example of the protection of plants against pathogens due to Si acting as a physical barrier is the pathosystem rice-Magnaporthe grisea, wherein the increase in resistance has been associated with the density of silicified cells present in the epidermis of the leaves, which act as a physical barrier to prevent penetration of the fungus (Datnofft et al., 2007). The hypothesis of the physical barrier was proposed and supported by Yoshida et al. (1962b) who reported the existence of a silica layer about 2.5 mm thick below the cuticle of the leaves of rice and said the second layer formed by Si on the cuticle could prevent penetration of M . grisea and thus reduce damage on the leaves of the plant. Furthermore, Volk et al. (1958) claim that Si can form complexes with organic compounds in the cell walls of epidermal cells, which can increase resistance to degradation by enzymes released by M. grisea. It was also suggested that Si may be associated with lignin-carbohydrate complexes present in the cell wall of epidermal cells of rice (Inanaga et al., 1995).
Kim et al. (2002) investigated some of the cytological features that may be associated with resistance to pathogen attack provided by Si and observed that the thickness of the epidermal cell wall was not significantly affected by the presence of Si, but the relationship between the thickness of the silica layer and the thickness of the epidermal cell wall was much higher in a cultivar which was identified as resistant than in one identified as susceptible. Although these authors concluded that fortification of epidermal cell walls could be the main cause of the reduction of injuries sustained in the leaves by pathogen attack, they did not assume that this was sufficient evidence to explain the impediment of fungal penetration in the leaves.
Kawamura and Ono (1948) reported that rice resistant to attack by Pyricularia oryzae had a lower number of lesions on the leaves and more silicified epidermal cells than susceptible crops, although Hashioka (1942) stated that the density of silicified cells in the epidermis of the leaves of rice was not proportional to the level of resistance to attack. From these results it was suggested that resistance to M. grisea in plants treated with Si is much more complex than a physical resistance to penetration due to the silicification of the cells or the double layer of Si formed in the cuticle (Datnofft et al., 2007). Recently, Heine et al. (2007) found that the accumulation of Si in the cell walls of the roots of squash and tomatoes did not represent a physical barrier to the spread of Pythium aphanidermatum but does contribute to increased resistance to the pathogen.
Resistance induced by Si
Rodrigues et al. (2003), studying the interaction of rice and Magnaporthe grisea at the cellular level when Si was applied, provided the first cytological evidence that Si mediated resistance to M. grisea is correlated with a specific cellular response in leaves which interferes with the propagation of the fungus. They found that fungal colonization was significantly reduced in samples of plants fertilized with Si, while those that were not had fungus that grew and colonized all tissues. The cytochemical marking of chitin revealed no material differences in the pattern of chitin localization over fungal cell walls in plant samples with Si and without it. In a subsequent study, Rodrigues et al.
(2004) found that leaf extracts of plants inoculated with M. grisea and fertilized with Si had higher amounts of phytoalexins than plants without Si. These results indicated that a limited production of chitinases can be one of the defense mechanisms of rice plants against the attack of the fungus and that compounds such as phenols and phytoalexins play a crucial role in the rice defense response against infection caused M. grisea, suggesting that Si plays an active role in rice resistance to the attack of the fungus, which is more complex than the formation of a physical barrier in the epidermis of the leaves. In addition to this, other researchers also observed an increase in the generation of superoxide radicals (O2-) in rice leaves treated with Si, the mechanism that prevents the growth of fungi (Datnofft et al., 2007).
Recent work with monocots and dicots confirmed an active role of Si in the natural stimulation of defense reactions of the plant (Walters and Bingham, 2007). An example was reported by Menzies et al. (1991a) who observed a negative correlation between concentrations of Si in the leaf tissues of cucumber plants and leaf area covered with colonies of Sphaerotheca fuliginea, for which the authors suggested that the increased resistance of the leaves of cucumber to pathogen attack was associated with enhanced epidermal cell walls produced by Si but also noted that the accumulation of Si around the colonies on the cucumber leaves affected fungal growth and diameter of the colonies. In another study, Menzies et al. (1991b) found a rapid accumulation of phenolic compounds in a large number of cells of plants of cucumber amended with Si and inoculated with S. fuliginea. Biochemical analysis of the extracts of the leaves of cucumber plants inoculated with the pathogen indicated the presence of flavonoids and phenolic acids which were specifically and strongly induced in a pattern typical of phytoalexins (Fawe et al., 1998). These findings support the theory that the resistance provided by Si to pathogen attack cannot be attributed solely to the presence of Si in the cell walls of the epidermal cells of the cucumber plant.
In cells of the root of cucumber plants, Si presented a rapid and extensive increase in electron density caused by the presence of phenolic compounds and antifungal activity against the pathogen Pythium ultimun which attacks the root (Chérif et al., 1992) as well as an increase in the activity of chitinase, peroxidases and polyphenoloxidase in the tissues of cucumber plants. In addition, extracts of plant tissues treated with Si and in the presence of P. ultimun showed a marked increase in the concentration of antifungal phenolics (Chérif et al., 1994). Other studies conducted on cucumber leaves investigating the process of infection of the plant showed that resistance to infection can be acquired by the expression of a protein rich in proline together with the presence of silica at the site of pathogen penetration (Kauss et al., 2003). The C-terminus of this protein contains lysine and arginine residues of high density, to which the catalysis is attributed in the formation of silica deposits localized at the site of vulnerability (Currie and Perry, 2007). It has been suggested that Si could act as a potentiator of defense responses or as an activator of protein-mediated cell signaling (Fauteux et al., 2005). Dann and Muir (2002) reported an increased activity of chitinase and b-1,3-glucanase in pea seeds inoculated with potassium silicate in plants that had been previously inoculated with Mycosphaerella pinodes. The number of lesions observed in tissues of plants with Si was lower than that of plants without Si. Other research in wheat (Bélanger et al., 2003) and rice (Rodrigues et al., 2004) on the defense mechanisms in which Si is involved in the presence of fungal pathogens, have also indicated that this element is capable of inducing biologically active defense agents, among which may be the increase in the production of glycosylated phenols and antimicrobial products such as diterpenoid phytoalexins (Currie and Perry, 2007).
As a step in this research, Ghanmi et al. (2004) formed a study of Arabidopsis thaliana in order to clarify the role of Si in plant-pathogen interactions. The results obtained were the first evidence that A. thaliana has the ability to absorb soluble Si which protects it from infection by the fungus Erysiphe cichoracearum. The results of this study corroborated recent observations in other species and helped support the theory that once absorbed by the plant, Si operates as a mediator of defense reactions and can control the activity of cell signaling systems. Hutcheson (1998) identified three classes of active defense mechanisms according to response: a) the primary response occurs in cells infected by the pathogen; b) the secondary response is induced by elicitors and limited to cells adjacent to the initial site of infection; c) the acquired systemic response is transmitted hormonally to all plant tissues.
The signals that direct the expression of defense responses of the plant are transmitted to the nucleus via activation of specific kinases and phosphatases cascades. Biotic stress responses are dependent on the Mitogen-activated protein kinases (MAP) that stimulate mitosis (mitogenic) (Takahashi, 1995; Nürnberger and Scheel, 2001; Tena et al., 2001; Morris, 2001; Zhang and Klessig, 2001; Fauteux et al., 2005). As protein kinases transmit information to the nucleus by phosphorylation of hydroxyl groups on amino acid residues, it has been suggested that Si may bind to the hydroxyl groups affecting the activity or the conformation of proteins. The mode of action of Si in signal transduction could also result from interactions with phosphorus or cations of micronutrients such as iron or manganese, in fact, metals play a structural role for many enzymes. Enzyme dysfunction may be due to excess essential metal species or the presence of toxic metal cations (Louie and Meade,1999), but it has not yet been established whether Si increases plant defenses by directly controlling the activity of proteins or indirectly through the sequestration of metal cations. After the pathogenattack, the infected tissue, through its defense reactions, synthesizes hormones and antimicrobial compounds such as salicylic acid and ethylene. It has been proposed that in a cell, Si controls the signaling events that guide the synthesis of these antimicrobial compounds, and could also control the generation of systemic signals. In this way, silicic acid, without being a second messenger, might play a role in resistance, both local and systemic (Fauteux et al., 2005). Si is a bioactive element in different biological systems, but its mode of action in plants is still not completely understood. This element has been shown to increase the expression of the natural defense mechanisms of plants and the accumulation of phytoalexins in monocots and dicots. The results reported for Si indicate that it may be acting locally through the induction of defense reactions and may also be contributing to systemic resistance through an increase in the production of stress hormones. However, the exact mechanism by which Si operates signaling in plants is still unclear. The evidence has shown that Si could act as an enhancer of the defense responses of plants or as an activator of protein-mediated cell signaling, implying that Si may interact with many key components of stress signaling systems in plants and direct induced resistance against fungal pathogens.
Literature cited
Arnon, D. and P. Stout. 1939. The essenciality of certain elements in minute quantity for plants with special reference to copper. Plant Physiol. 14, 371-375. [ Links ]
Bélanger, R., N. Benhamou, and J. Menzies. 2003. Cytological evidence of an active role of silicon in wheat resistance to powdery mildew (Blumeria graminis f. sp. tritici). Phytopathology 93, 402-412. [ Links ]
Chen, Y. 1990. Characteristics of silicon uptaking and accumulation in rice. J. Guizhou Agric. Sci. 6. 37-40. [ Links ]
Chérif, M., N. Benhamou, J. Menzies, and R. Bélanger. 1992. Silicon induced resistance in cucumber plants against Pythium ultimun. Physiol. Mol. Plant Pathol. 41, 411-425. [ Links ]
Chérif, M., A. Asselin, and R. Bélanger. 1994. Defense responses induced by soluble silicon in cucumber roots infected by Pythium spp. Phytopathology 84, 236-242. [ Links ]
Currie, H. and C. Perry. 2007. Silica in plants: biological, biochemical and chemical studies. Ann. Bot. 100, 1383-1389. [ Links ]
Dann, E. and S. Muir. 2002. Peas grown in media with elevated plant-available silicon levels have higher activities of chitinases and b-1,3-glucanase, are less susceptible to a fungal leaf spot pathogen and accumulate more foliar silicon. Austral. Plant Pathol. 31, 9-13. [ Links ]
Datnofft, L., N. Raid, G. Snyder, and D. Jones. 1991. Effect of calcium silicate on blast and brown spot intensities and yields of rice. Plant Dis. 75, 729-732. [ Links ]
Datnofft, L., W. Elmer, and D. Huber. 2007. Mineral nutrition and plant disease. The American Phytopathological Society, St. Paul, MN. [ Links ]
Epstein, E. 1994. The anomaly of silicon in plant biology. Proc. Natl. Acad. Sci. 91, 11-17. [ Links ]
Epstein, E. 1999. Silicon. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 641-64. [ Links ]
Fauteux, F., W. Rémus-Borel, J. Menzies, and R. Bélanger. 2005. Silicon and plant disease resistance against pathogenic fungi. FEMS Microbiol. Lett. 249, 1-6. [ Links ]
Fawe, A., M. Abou-Zaid, J. Menzies, and R. Bélanger. 1998. Siliconmediated accumulation of flavonoid phytoalexins in Cucumber. Phytopathology 88, 396-401. [ Links ]
French-Monar, R., F. Avila, G, Korndorfer, and L. Datnoff. 2010. Silicon suppresses Phytophthora blight development on bell pepper. J. Phytopathol. 158, 554-560. [ Links ]
Ghanmi, G., N. McNallya, G. Menzies, and R. Bélanger. 2004. Powdery mildew of Arabidopsis thaliana: a pathosystem for exploring the role of silicon in plant–microbe interactions. Physiol. Mol. Plant Pathol. 64, 189-199. [ Links ]
Gillman, J., D. Zlesak, and J. Smith, 2003. Applications of potassium silicate decrease black spot infection of Rosa hybrida 'Meilpelta'. HortScience 38, 1144-1147. [ Links ]
Harrison, C. 1996. Evidence for intramineral macromolecules containing protein from plant silicas. Phytochemistry 41, 37-42. [ Links ]
Hashioka, Y. 1942. Studies on rice blast disease in the tropics. (I) Anatomical comparison of leaf epidermis of Formosan rice with that of Taiwan rice from plant pathological viewpoints. Agric. Hort. 17, 846-852. [ Links ]
Heine, G., G. Tikum, and W. Horst. 2007. The effect of silicon on the infection by and spread of Pythium aphanidermatum in single roots of tomato and bitter gourd. J. Exp. Bot. 58(3), 569-577. [ Links ]
Hodson, M., P. White, A. Mead, and M. Broadley. 2005. Phylogenetic variation in the silicon composition of plants. Ann. Bot. 96, 1027-1046. [ Links ]
Huber, D. 1997. Manejo de la nutrición para el combate de patógenos de plantas. Agron. Costarr. 21, 99-102. [ Links ]
Huber, D. and R. Graham. 1999. The role of nutrition in crops resistance and tolerance to disease. pp. 169-204. In: Rengel, Z. (ed.). Mineral nutrition of crops: fundamental mechanism and implications. The Haworth Press, Binghamton, NY. [ Links ]
Huber, D. and S. Haneklaus. 2007. Managing nutrition to control plant disease. Landbauforschung Volkenrode 57, 313-322. [ Links ]
Hutcheson, S. 1998. Current concepts of active defense in plants. Annu. Rev. Phytopathol. 36, 59-90. [ Links ]
Inanaga, S., A. Okasaka, and S. Tanaka. 1995. Does silicon exist in association with organic compounds in rice plant? Jpn. J. Soil Sci. Plant Nutr. 11, 111-117. [ Links ]
Jones, L. and K. Handreck. 1967. Silica in soils, plants, and animals. Adv. Agron. 19, 107-149. [ Links ]
Kauss, K., R. Franke, S. Gilbert, A. Dietrich, and N. Kroger. 2003. Silica deposition by a strongly cationic proline-rich protein from systemically resistant cucumber plants. Plant J. 33, 87-95. [ Links ]
Kawamura, E. and K. Ono. 1948. Study on the relation between the pre–infection behavior of rice blast fungus, Piricularia oryzae, and water droplets on rice plant leaves. Bull. Natl. Agr. Exp. Sta. 4, 1-12. [ Links ]
Kawashima, R. 1927. Influence of silica on rice blast disease. Jpn. J. Soil Sci. Plant Nutr. 1, 86-91. [ Links ]
Kim, S., W. Kim, E. Park, and D. Choi. 2002. Silicon-induced cell wall fortification of rice leaves: a possible cellular mechanism of enhanced host resistance to blast. Phytopathology 92, 1095-1103. [ Links ]
Kolesnikov, M. and V. Gins. 2001. Forms of silicon in medicinal plants. Appl. Biochem. Microbiol. 37, 524-527. [ Links ]
Kunoh, H. and H. Ishizaki. 1975. Silicon levels near penetration sites of fungi on wheat, barley, cucumber and morning glory leaves. Physiol. Plant Pathol. 5, 283-287. [ Links ]
Leusch, H. and H. Buchenauer. 1989. Effect of soil treatments with silica-rich lime fertilizers and sodium trisilicate on the incidence of wheat by Erysiphe graminis and Septoria nodorum depending on the form of N-fertilizer. J. Plant Dis. Prot. 96, 154-172. [ Links ]
Louie, A. and T. Meade. 1999. Metal complexes as enzyme inhibitors. Chem. Rev. 99, 2711-2734. [ Links ]
Ma, J., K. Nishimura, and E. Takahashi. 1989. Effect of silicon on the growth of rice plant at different growth stages. Jpn. J. Soil. Sci. Plant Nutr. 35, 347-356. [ Links ]
Ma, J. and E. Takahashi. 2002. Soil, fertilizer, and plant silicon research in Japan. Elsevier Science, Amsterdam, The Netherland. [ Links ]
Ma, J. and N. Yamaji. 2006. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 11(8), 392-397. [ Links ]
Ma, J. and N. Yamaji. 2008. Functions and transport of silicon in plants. Cell. Mol. Life Sci. 65, 3049-3057. [ Links ]
Marschner, H. 2002. Relationships between mineral nutrition and plant diseases and pests. pp. 436-460. In: Marschner, H. (ed.). Mineral nutrition of higher plants, Elsevier Science, New York, NY. [ Links ]
McKeague, J. and M. Cline. 1963. Silica in soils. Adv. Agron. 15, 339-396. [ Links ]
Menzies, J., D. Ehret, A. Glass, and A. Samuels. 1991a. The influence of silicon on cytological interactions between Sphaerotheca fuliginea and Cucumis sativus. Physiol. Mol. Plant. Pathol. 39, 403-414. [ Links ]
Menzies, J., D. Ehret, A. Glass, T. Helmer, T. Koch, and F. Seywerd. 1991b. Effects of soluble silicon on the parasitic fitness of Sphaerotheca fuliginea on Cucumis sativus. Phytopathology 81, 84-88. [ Links ]
Morris, P. 2001. MAP kinase signal transduction pathways in plants. New Phytologist 151, 67-89. [ Links ]
Munévar, F. 2004. Relación entre la nutrición y las enfermedades de las plantas. Palmas 25, (Supp.) 171-178. [ Links ]
Nürnberger, T. and D. Scheel. 2001. Signal transmission in the plant immune response. Trends Plant Sci. 6, 372-379. [ Links ]
Onodera, I. 1917. Chemical studies on rice blast. J. Sci. Agric. Soc. 180, 606-617. [ Links ]
Perry, C. and Y. Lu. 1992. Preparation of silicas from silicon complexes: role of cellulose in polymerisation and aggregation control. Faraday Trans. 88, 2915-2921. [ Links ]
Perry, C. and K. Tucker. 2000. Biosilicification: the role of the organic matrix in the structure control. J. Biol. Inorg. Chem. 5, 537-550. [ Links ]
Pilon-Smits, E., C. Quinn, W. Tapken, M. Malagoli, and M. Shiavon. 2009. Physiological functions of beneficial elements. Curr. Opin. Plant Biol. 12, 267-274. [ Links ]
Prychid, C., P. Rudall, and M. Gregory. 2004. Systematics and biology of silica bodies in monocotyledons. Bot. Rev. 69, 377-440. [ Links ]
Reifenberg, A. and S. Buckwold. 1954. The release of silica from soils by the orthophosphate anion. J. Soil. Sci. 5. 106-115. [ Links ]
Richmond, K. and M. Sussman. 2003. Got silicon? The non-essential beneficial plant nutrient. Curr. Opin. Plant Biol. 6, 268-272. [ Links ]
Rodrigues, F., N. Benhamou, L. Datnoff, J. Jones, and R. Bélanger. 2003. Ultrastructural and cytochemical aspects of siliconmediated rice blast resistance. Phytopathology 93, 535-546. [ Links ]
Rodrigues, F., D. McNally, L. Datnoff, J. Jones, C. Labbé, N. Benhamou, J. Menzies, and R. Bélanger. 2004. Silicon enhances the accumulation of diterpenoid phytoalexinsin rice: a potential mechanism for blast resistance. Phytopathology 94, 177-183. [ Links ]
Rodgers-Gray, B. and M. Shaw. 2000. Substantial reductions in winter wheat diseases caused by addition of rice straw but not manure to soil. Plant Pathol. 49, 590-599. [ Links ]
Rodgers-Gray, B. and M. Shaw. 2004. Effects of straw and silicon soil amendments on some foliar and stem-base diseases in pot-grown winter wheat. Plant Pathol. 53, 733-740. [ Links ]
Savant, N., G. Snyder, and L. Datnoff. 1997a. Depletion pf plantavailable silicon in soils: A possible cause of declining rice yields. Commun. Soil Sci. Plant Anal. 28, 1245-1252. [ Links ]
Savant, N., G. Snyder, and L. Datnoff. 1997b. Silicon management and sustainable rice production. Adv. Agron. 58, 151-199. [ Links ]
Seebold, K., L. Datnoff, F. Correa-Victoria, T, Kucharek, and G. Snyder. 2000. Effect of silicon rate and host resistance on blast, scald, and yield of upland rice. Plant Dis. 84, 871-876. [ Links ]
Solomon, P., K. Tan, and R. Oliver. 2003. The nutrient supply of pathogenic fungi; a fertile field for study. Mol. Plant Pathol. 4, 203-210. [ Links ]
Sommer, M., Kaczorek, Y. Kuzyakov, and J. Breuer. 2006. Silicon pools and fluxes in soils and landscapes – a review. J. Plant Nutr. Soil Sci. 2, 99-122. [ Links ]
Sun, X., Y. Sun, C. Zhang, Z. Song, J. Chen, J. Bai, Y. Cui, and C. Zhang. 1994. The mechanism of corn stalk rot control by application of potassic and siliceous fertilizers. Acta Phytophyl. Sin. 21, 102-108. [ Links ]
Sun, W., Y. Liang, and Y. Yang. 2002. Influences if silicon and inoculation with Colletotrichum lagenarium on peroxidase activity in leaves of cucumber and their relation to resistance to anthracnose. Sci. Agric. Sin. 35, 1560-1564. [ Links ]
Takahashi, E., J. Ma, and Y. Miyake. 1990. The possibility of as an essential element for higher plants. Comments Agric. Food Chem. 2, 99-122. [ Links ]
Takahashi, E. 1995. Uptake mode and physiological functions of silica. Sci. Rice Plant 2, 58-71. [ Links ]
Tena, T., T. Asai, W. Chiu, and J. Sheen. 2001. Plant mitogenactivated protein kinase signaling cascades. Curr. Opin. Plant Biol. 4, 392-400. [ Links ]
Turner, P. 2003. The role of nutrition in desease control. pp. 181-190. In: Fairhurst, Th. and R. Härdter (eds.). The oil palm. Management for large and sustainable yields. Potash and Phosphate Institute of Canada (ESEAP), Watten Estate, Singapore. [ Links ]
Volk, R., R. Kahn, and R. Weintraub. 1958. Silicon content of the rice plant as a factor influencing its resistance to infection by the rice blast fungus, Picularia oryzae. Phytopathology 48, 179-184. [ Links ]
Walters, D. and I. Bingham. 2007. Influence of nutrition on disease development caused byfungal pathogens: implications for plant disease control. Ann. Appl. Biol. 151, 307-324. [ Links ]
Winslow, M. 1995. Silicon: A new macronutrient deficiency en upland rice. CIAT Work. Doc. No. 149. International Center for Tropical Agriculture (CIAT), Cali, Colombia. [ Links ]
Yoshida, S., Y. Ohnishi, and K. Kitagishi. 1962a. Histochemistry of silicon in rice plant II. Soil Sci. Plant Nutr. 8(2), 36-41. [ Links ]
Yoshida, S., Y. Ohnishi, and K. Kitagishi. 1962b. Chemical forms, mobility, and deposition in the rice plant. Soil Sci. Plant Nutr. 8(3), 107-113. [ Links ]
Zhang, S. and D. Klessig. 2001. MAPK cascades in plant defense signaling. Trends in Plant Science 6, 520-527. [ Links ]




![Biological nitrogen fixation by Rhizobium sp. native gliricidia (Gliricidia sepium [Jacq.] Kunth ex Walp.) under greenhouse conditions](/img/en/prev.gif)