Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Agronomía Colombiana
Print version ISSN 0120-9965
Agron. colomb. vol.30 no.1 Bogotá Jan./Apr. 2012
1 Universidad de la Amazonia, Florencia, Caquetá and Instituto de Ciencia y Tecnología de Alimentos (ICTA), Universidad Nacional de Colombia. Bogota (Colombia).
2 Instituto Amazónico de Investigaciones Científicas (Sinchi) and Instituto de Ciencia y Tecnología de Alimentos (ICTA), Universidad Nacional de Colombia. Bogota (Colombia).
3 Corresponding author. cehernandez@uniamazonia.edu.co
Received for publication: 11 August, 2010. Accepted for publication: 1 March, 2012.
ABSTRACT
Studies of growth and optimal harvest time of cupuaçu are vital to ensure fruit quality and reduce post-harvest losses. This studied looked at growth and fruit development of cupuaçu from fruit set to ripening. The measurements analyzed included diameter (longitudinal and equatorial), fresh and dry weight, color, pH, titratable acidity (TA), total soluble solids (TSS) and respiratory rate (RR). Three sigmoid states were observed during fruit growth: cell division (S1), maximum growth (S2), which corresponds to cell expansion and growth stabilization and maturation (S3). The time between fruit set and physiological maturity was 117 days. The cupuaçu fruit reached physiological maturity when it showed changes in pulp color (H* = 97.1±1.8°), which coincided with a TSS of about 5.7±0.8°Brix, which were a good index of maturity along with days after fruit set. The respiration pattern of the cupuaçu fruit was climacteric, with a peak of 156.24±42.5 mg CO2 kg-1 h-1 124 days after fruit set. No ethylene was detected before harvest, but was detected in some fruits postharvest.
Key words: Hue angle (H*), maturity index, logistic model, respiratory rate.
RESUMEN
Los estudios de crecimiento y momento óptimo de cosecha de copoazú son importantes para garantizar la calidad del fruto y reducir las pérdidas poscosecha. Se estudió el crecimiento y desarrollo del fruto de copoazú, desde el cuajado hasta la madurez de consumo. Fueron analizados diámetro (longitudinal y ecuatorial), peso fresco y seco, color, pH, acidez titulable (AT), sólidos solubles totales (SST) e intensidad respiratoria (IR). Fueron reconocidos tres estados tipo sigmoide en el crecimiento del fruto: división celular (E1), máximo crecimiento (E2), el cual corresponde a la expansión celular, y estabilización del crecimiento y maduración (E3). El tiempo transcurrido entre el cuajado del fruto y la madurez fisiológica fue 117 días. El fruto de copoazú alcanzó la madurez fisiológica cuando mostró cambios en el color de la pulpa (H*=97,1±1,8°), que coincidieron con unos SST alrededor de 5,7±0,8°Brix, los cuales constituyeron un buen índice de madurez junto con los días después del cuajado del fruto. El patrón respiratorio del fruto de copoazú fue de tipo climatérico, con un pico de 156,24±42,5 mg CO2 kg-1 h-1 124 días después del cuajado. No fue detectado etileno antes de la cosecha, pero si fue detectado en algunos frutos en poscosecha.
Palabras clave: ángulo Hué (H*), índice de madurez, modelo logístico, intensidad respiratoria.
Introduction
The cupuaçu fruit (Theobroma grandiflorum [Willd. ex Spreng.] Schum.), Family Malvaceae (Alverson et al., 1999), has a high economic potential due to its agribusiness, shown by its high percentage of acidity and vitamin C in the pulp and high protein and fat content in the seed (Carvalho et al., 1999). This species has thick fleshy fruits (Ibarra-Manríquez and Cornejo-Tenorio, 2010), with a hard epicarp: woody, green skin, covered with a dusty, ferruginous layer (Carvalho et al., 1999). The fruits are usually collected from the ground, as the skin color does not change when ripe (Rojas et al., 1998). This practice causes damage due to contamination and rodents. For this reason, studies of growth and optimal harvest time are important to ensure fruit quality and reduce post-harvest losses.
Fruits of the genus Theobroma (cupuaçu and macambo (T. bicolor)) are classified as non-climacteric (Carvalho et al., 1999, Hernández et al., 2006), however, the postharvest physiology of cupuaçu fruit should be studied further, taking into account chemical, physical and sensory developments during the two days after harvest (Carvalho et al., 1999). In the pulp of these fruits at maturation, the TA decreases, pH and TSS increase and color changes (from white to creamy yellow in cupuaçu, and light yellow to dark yellow in Maraco) (Hernández et al., 2006).
In the western colombian Amazon (Caqueta), there have been no studies of growth and development of cupuaçu and there are no maturity indices. Therefore, the objective of this study was to analyze the growth and development of the fruit; set collection parameters for cupuaçu in the western colombian Amazon, in order to reduce losses due to inadequate harvests and improve the quality of the products obtained from the fruit.
Materials and methods
The study was conducted on the farm Estefania (1°39'49.8" N and 75°36'55.7" W) in Florencia (Caqueta), western Colombian Amazon. The environmental conditions were: altitude 332 m, mean annual temperature 25.03°C, 86.1% RH, precipitation 3,623.8 mm and sunshine 1,465.4 h year-1.
The ecotype employed corresponds to accession A4 from the C.I. Macagual germplasm bank. According to Escobar et al. (2009), this ecotype has large oval fruit with a sharp apex and base; the pulp is yellow. The ripe fruit longitudinal diameter varies between 16 and 22 cm (18.7 cm on average), the equatorial diameter ranges from 10.1 to 12.2 cm (mean 10.58 cm) and weighs approximately 1320 g.
The 185 recently set fruits were labeled (4.12±0.20 cm in longitudinal diameter and 1.24±0.12 cm in equatorial diameter) from 23 trees on a 4.5 year old plantation and were monitored from fruit set until four days after natural abscission. Samples were taken every 2 weeks.
The collected fruits were placed in damp newspaper and transported at room temperature in closed plastic bags to the Nutrition Laboratory, Universidad de la Amazonia (Florencia). The time between collection and the measurements was one hour. Three replications were observed separately for longitudinal and equatorial diameter (LD and ED, respectively) measured with a Vernier caliper, 0.01 cm precision (model 700-103BPC-600B, General Supply Corporation, Jackson, MA). The fresh weight (FW) was measured with a 0.01 g precision balance (model BC2200C, Precision, Dietikon, Switzerland). The dry weight (DW) was determined by placing each of the components of the fruit in an oven at 70°C until constant weight. The skin color was measured at two opposite points along the fruit equator after brush removal of the ferruginous layer, pulp and seed color were also measured. In all cases, the coordinates L* C* H* were employed with a Hunter Lab colorimeter miniscan XE Plus (Illuminant D65, 2° observer).
The respiratory rate (RR) of the fruit was determined according to the static method (Kader, 2002a) by confining individual fruits for 2.5 h at 27°C and 98,08 kPa. The CO2 and ethylene production were measured by gas chromatography using a gas chromatograph (GC) Agilent 4890D coupled with an integrator hp 3395/3396. For measurements of CO2 and C2H4, the GC was coupled to a thermal conductivity detector (TCD) and a flame ionization detector (FID), respectively. The temperatures of the oven, the injector and the TCD were 30, 50 and 300°C, respectively, for the CO2 determinations. For measurement of ethylene production, the corresponding temperatures for the oven, the injector and FID were 30, 50 and 250°C, respectively. The gas flow rates (helium, synthetic air and hydrogen) were 5·10-7, 5·10-6 and 8·10-7 m3 s-1. The column was calibrated with a certified standard of 2% CO2 and 4.175·10-4 mol m-3 ethylene (AGA, Bogotá, Colombia). The total soluble solids (TSS) was measured directly from the juice of the pulp of each fruit with a 103 bp portable Atago refractometer (Atago, Japan). Subsequently, the same fruit pulp was homogenized, and 5 g were mixed with 30 cm3 of distilled water, the pH of the resulting mixture was measured with a Consort C931 electrode electrochemical analyzer (Turnhout, Belgium) before being brought to a pH of 8.1 with a 0.1 N NaOH solution using the titratable acidity method (TA) (Mercado- Silva et al., 1998). TA was reported in percentage by weight of citric acid and the ratio of TSS and TA (maturity ratio (MR)) was tabulated as TSS/citric acid (%).
The fruit growth traits were fitted to a logistic model:
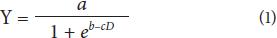
Where the coefficient is the maximum reached by the fruit size, b controls the speed of growth, c affects the slope of the growth curve and D is the time in days after fruit set (DAS). Logistic regression models were estimated using analytical software Statistix 9.0 (Analytical Software, 2008) according to Garriz et al. (2005) and Barrera et al. (2008). The fitness of the logistic model was evaluated using the value of R2 and the mean square residual (Garriz et al., 2005). Other variables were subjected to ANOVA with time as a factor of growth, previously checking randomness, normality and homoscedasticity using Statgraphics® Plus (Statgraphics, 2000). Means were compared with the multiple ranges Tukey test, HSD at 95%.
Results
Fruit growth
Cupuaçu's growth conformed to a simple sigmoid curve for the diameters and fresh weight (Tab. 1, Fig. 1A to 1C). For dry weight, the logistic model achieved a good fit (R2 = 0.93), but did not present a sigmoidal shape. Maximum growth was reached about 83 d after fruit set. Logistic regression was a good fit for growth traits and low mean square residual (R2≤0.85, P≤0.001).
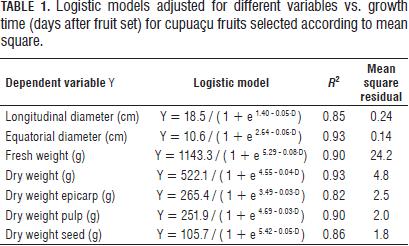
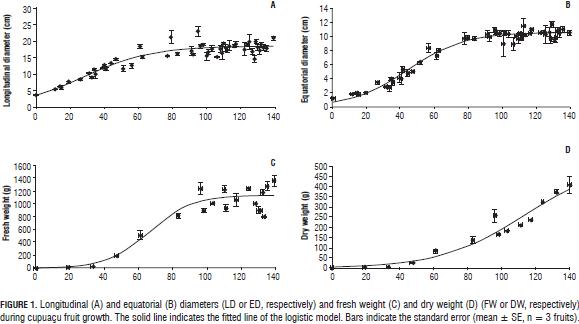
In growth models for fresh and dry weight three states were identified: S1 beginning with fruit set and where there will be a certain number of cell divisions post-set, according to statistical analysis S1 lasts up to 33 d. S2 corresponds to the cell elongation stage; is from 33 to 83 d. And a final step, S3 corresponding to the stabilization step where fruit growth reached its final size at 96 d (Fig. 1A through 1C). The natural abscission presented at 139 DAS.
The residual degrees of freedom were 258 for the longitudinal and equatorial diameters, 118 for fresh weight, 65 for dry weight and 25 for dry weight of the epicarp, pulp and seed. The terms were significant (P≤0.001).
The model grows slowly in S1 for FW and DW (Fig. 1C and 1D), and fast for LD and ED (Fig. 1A and 1B). The four morphological variables increased exponentially during the second state (S2) (Fig. 1A-1D). The fresh weight (FW) was less constant in S3 (Fig. 1C). The longitudinal and equatorial diameters and fresh weight were fitted to a simple sigmoid curve with high correlation coefficients (R2=0.85) (Fig. 1A to 1C and Tab. 2) and statistical significance (P≤0.001).
Color
The pulp remained white for S1, S2 and the beginning of S3 (L* = 75.8±2.8, C* = 8.0±0.4, H* = 98.0±1.7°). During S3, the pulp became yellow and intensified the decrease of H * and the increase of C * until day 132 (Fig. 2B). During S3, the pulp presented the lowest H * at day 143 (90.4±1.2°); the highest C * (23.0±2.7) was seen at day 140 (Fig. 2B).
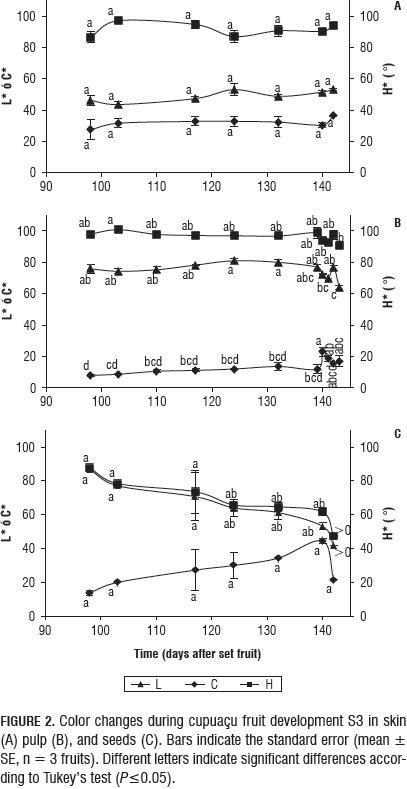
The L* (81.0±1.2) of the pulp was highest at day 124 (Fig. 2B). Following natural fruit abscission, L* increased significantly (P≤0.01) for the 142 d, then declined until day 143 (Fig. 2B).
The seed remained a similar color to the pulp for S1, S2 and the beginning of S3 (L* = 86.8±1.2, C* = 13.9±1.5, H* = 87.7±1.0°). During S3, the seed became brown with decreasing H* until day 140 (Fig. 2C). In the seed, H* (47.5±0.4°) and L* (42.0±0.01) showed a significant decrease (P≤0.05 and P≤0.01) at day 142, with respect to days 98 and 117 (Fig. 2C).
The color of the skin beneath the ferruginous layer became yellower during S3, and more intense and bright with the decrease of H* and the increase of C* and L* at day 124 (Fig. 2A). For the skin, H*, L* and C* did not present significant changes in S3 (Fig. 2A).
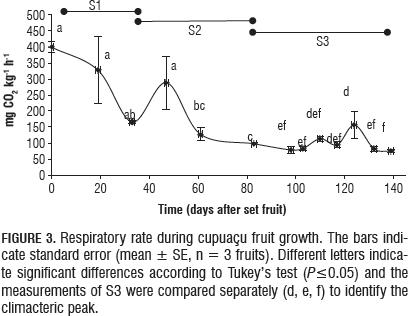
Respiration
The RR (mg CO2 kg-1 h-1) was high (399.37±17.96 mg) in S1 (Fig. 3). Although the beginning of S2 presented a transient climacteric, the RR decreased to 97.37±3.03 mg (Fig. 3). Finally, during S3 a peak climacteric appeared (156.24±42.5 mg) at day 124 (Fig. 3). The increase in the climacteric peak was significant (P≤0.05), but did not present a detectable ethylene production before natural fruit abscission.
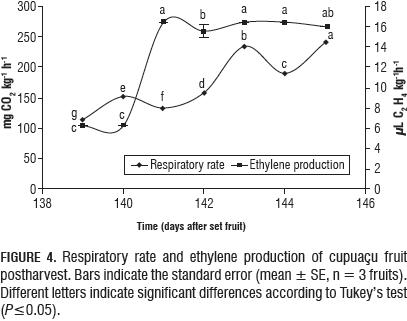
After natural fruit abscission, the RR increased from 114.15 to 234.65 mg over four days (Fig. 4), and presented parallel detectable emission of ethylene (6.23 to 16.47 L C2H4 kg m-1 h-1) (Fig. 4).
Other quality traits
During the climacteric peak (124 DAS), TA decreased to 2.0±0.2%, then increased to 3.1±0.2% at the end of S3 (Fig. 5A). The pH showed opposite behavior from TA, with a slight increase during the climacteric peak (Fig. 5A), no significant changes for either.
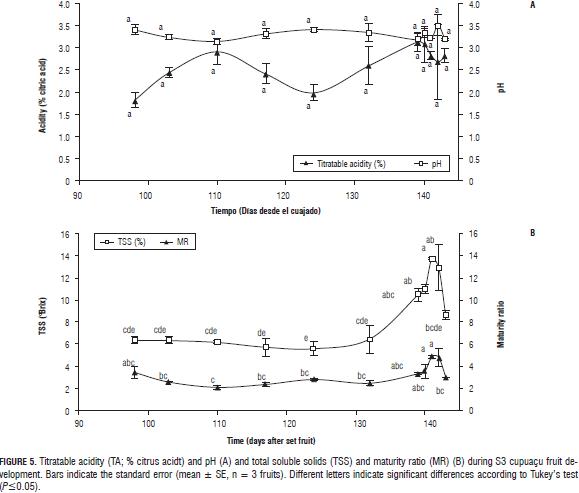
MR decreased between 98 and 110 DAS, from 3.5 to 2.1, then increased to 3.1 at the end of S3 (Fig. 5B). The TSS showed a significant increase (P≤0.05) 139 DAS from 6.5±1.3 to 10.5±0.6°Brix (Fig. 5B).
After natural abscission, TSS and MR increased (10.5±0.6 to 13.7±0.1°Brix and 3.3 to 4.9, respectively) over 2 d, and then decreased (Fig. 5B).
Discussion
Fruit growth
The fruit had a single sigmoid growth for 139 d, which coincides with Rojas et al. (1998) for Caqueta (140 d) and Calzavara et al. (1984) for Brazil (120-135 d). In contrast, Hernández et al. (2006) reported 240 d in Guaviare. The difference in development time between northeastern Amazon (Guaviare) and western Amazon (Caqueta) could be caused by climatic factors or the existence of genetic diversity (Hernández et al., 2007).
Models for cupuaçu fruit growth, Caqueta (Tab. 2) differ from those reported by Hernández et al., (2006) for Guaviare. LD, ED and FW were fitted to a logistic model used for arazá (Hernández et al., 2007), Amazonian Ajis (Barrera et al., 2008), Abbé Fetel pears (Garriz et al., 2005), among others.
According to Barceló et al. (2005), the change in DW and FW in S1 is caused by the increase in cell number, likewise the change in fruit LD and is associated with the cell division process. In the exponential increase in S2, all morphological variables are associated with cell elongation, accumulation of reserve photoassimilates and water, increasing size of the vacuoles and the beginning of the accumulation of organic acids, sugars and other components. During S3, the fruit dimensions and FW stabilized, but DW continued to increase (Fig. 1D), associated with increased translocation of assimilates from the leaves.
Color
None of the color components of the skin changed significantly during S3. Indicating that the external color of the fruit cannot be used as a harvest index as in breadfruit (Artocarpus altilis) (Worrell et al., 1998).
The pulp color change from white to cream yellow during S3 was also observed by Hernández et al. (2006). Pulp color changes have been observed in early stages of growth and maturity for other fruits such as maraco (Hernández et al., 2006) and arazá (Galvis and Hernández, 1993).
The C* of the pulp increased when H* decreased at 132 d. This color behavior has also performed in Sweet pepper cv. Domino (Tadesse et al., 2002). The change in color of the pulp may be associated with increased synthesis of carotenoids; cupuaçu pulp contains 127.9±4.54 g/100 g (Sousa et al., 2011). In most fruits, conversion of chloroplasts to chromoplasts is accompanied by synthesis of one or several kinds of pigments, normally anthocyanins or carotenoids (Hobson, 1999; Kays and Paull, 2004).
Importantly, different colors may have the same value of chroma (C*) and therefore, this is not an adequate maturity indicator as it is in peppers (López and Gómez, 2000).
Respiration
The high respiration rate in early S1 is due to a high degradation of substances through oxide reduction reactions, to achieve the necessary energy for the processes of development (growth and differentiation) and cell maintenance (Barcelo et al., 2005; Wills et al., 1998). The respiratory rate at the beginning of S1 is almost four times lower than that reported by Hernández and Galvis (1994), who used the dynamic method (Kader, 2002a). At the beginning of S2, a transient climacteric was seen, which has been observed in Amazonian Ajis (Barrera et al., 2008) and in previous studies on cupuaçu (Hernández et al., 2006) and may suggest a mechanism that triggers metabolic processes after generating changes associated with physiological maturity of the fruit (Barrera et al., 2008).
Climacteric respiration was seen after 124 d (S3) without a detectable production of ethylene. This behavior matches that observed in guava fruit, cherimoya and avocado, where climacteric respiration significantly precedes increased ethylene synthesis (Kays and Paull, 2004).
The climacteric peak observed is 4.5 times lower than that reported by Hernández and Galvis (1994) for Guaviare, which came 180 d after fruit set. That is, the climacteric peak for cupuaçu in Caquetá occurred 56 days earlier than in the study on Guaviare. This difference is associated with a shorter growth cycle for cupuaçu, Caqueta.
Cupuaçu presents a climacteric behavior, a result that contrasts with previous studies that classified it as nonclimacteric (Carvalho et al., 1999, Hernández et al., 2006). The climacteric pattern makes it advisable to harvest 117 d after fruit set when the fruit has reached physiological maturity and the respiratory rate is minimal, just before the climacteric peak. However, this parameter must be managed with other, more stable parameters, taking into account the age of the fruit may vary depending on environmental factors and the cultivar.
The respiratory model of cupuaçu matches that of the peach, which has similar levels of CO2 production in the three stages of growth (Seymour et al., 1993). Considering the categories proposed by Kader (2002b), cupuaçu can be classified as a fruit with an extremely high respiratory rate. However, other fruits have higher respiratory intensities, such as arazá (Eugenia stipitata) (Hernández et al., 2007) and acerola (Malpighia emarginata) (Carrington and King, 2002). The climacteric behavior of cupuaçu differs from other fruits of the genus Theobroma such as cacao (Kader, 2002b; Kays and Paull, 2004) and Maraco (Hernández et al., 2006), which are classified as non-climacteric.
Ethylene production of cupuaçu resembles that of the peach in the late S3 state (= 20 mL C2H4 kg-1 h-1) and kiwi fruit during ripening (60-80 mL C2H4 kg-1 h-1) (Seymour et al., 1993). Given the categories proposed by Kader (2002b), cupuaçu can be classified as a fruit with a moderate to high ethylene production rate. The increase in the respiratory rate after abscission may be naturally associated with the processes that are triggered due to the production of ethylene and lead to senescence.
According to the categories established by Kader (2002b) and taking into account the data of extremely high respiratory rate and high ethylene production, the perishability of the cupuaçu fruit could be estimated at 2-4 weeks, but studies should be done in this regard.
Other quality traits
The decrease in TA and increased pH during the climacteric peak indicated consumption of organic acids as respiratory substrates. Increased TA after this event possibly indicates new synthesis of organic acids or an effect on concentration by reduced fresh weight (Fig. 1C). The decline in MR between 98 and 110 d is a result of increased TA and the stable behavior of TSS before the climacteric peak, because TSS increased significantly only after this point. This behavior for TA differs from that previously observed in cupuaçu and maraco (Hernández et al., 2006), where TA decreased at the end of S3.
The fluctuation in TA and the stable behavior of TSS suggest that acids are used more than sugars for respiration. The increase in TSS after the climacteric peak may indicate that this process triggers a conversion mechanism of starch into sugars. The increase in TSS and acid at the end of the S3 has also been observed in guava (Bulk et al., 1996, Mercado- Silva et al., 1998) and feijoa (Rodríguez et al., 2006). The Cupuaçu pulp presented a significant TA and a fairly stable but low pH. According to Salisbury and Ross (2006), the low pH may be associated with two aspects: 1) high acid contents stored in the vacuole and 2) with the growth of the cells, which requires low pH levels.
In general, the increased pH and TSS after the natural fruit abscission is due to the respiration process performed to obtain the energy required for metabolic functions; behavior consistent with the results of this study, since as mentioned before, after abscission of the fruit, respiratory intensity of cupuaçu increased (Fig. 2C).
Conclusions
In climatic conditions of the western Colombian Amazon, the cupuaçu fruit reaches physiological maturity 117 days after fruit set. When MR is 2.4. The diameters and the fresh weight exhibited a single sigmoid growth model. The cupuaçu fruit behaved as a climacteric fruit, with a peak of respiratory activity at 156.24±42.5 mg CO2 kg-1 h-1, 124 d after fruit set. However, no detectable ethylene emission presented pre-harvest.
During S3, the skin color did not change significantly, however, the pulp changed from white to cream yellow, showing that this change can be used as a maturity index.
After natural abscission, the cupuaçu fruit decreased all physicochemical variables, RR increased and emission of ethylene slowed, but not in all fruits. The ethylene production levels ranged from 6.23 to 16.47 mL C2H4 kg-1 h-1 over a period of four days.
The parameters for cupuaçu harvest in the western Colombian Amazon may be the days after fruit set (117 d), along with pulp color around H* = 97.1±1.8° and a total soluble solids value of at least 5.7±0.8°Brix.
Acknowledgements
Thanks to the Instituto Amazónico de Investigaciones Científicas-Sinchi (Amazonian Scientific Research Institute), to Asohofrucol and the Ministerio de Ambiente, Vivienda y Desarrollo Territorial (Ministry of Environment, Housing and Territorial Development) for the financial support granted (101-2/06 Project).
Literature cited
Alverson, W.S., B.A. Whitlock, R. Nyffeler, C. Bayer, and D.A. Baum. 1999. Phylogeny of the core Malvales: evidence from sequence data. Amer. J. Bot. 89, 1474-1486. [ Links ]
Analytical Software. 2008. STATISTIX for Windows Versión 9. Tallahassee, FL. [ Links ]
Barceló, J., R. Nicolás, B. Sabater, and R. Sánchez. 2005. Fisiología vegetal. Ediciones Pirámide, Madrid. [ Links ]
Barrera, J.A., M.S. Hernández, L.M. Melgarejo, O. Martínez, and J.P. Fernández-Trujillo. 2008. Physiological behavior and quality traits during fruit growth and ripenning of four Amazonic hot pepper accessions. J. Sci. Food Agric. 88, 847-857. [ Links ]
Bulk, R.E.E., E.F.E. Babiker, and A.H.E. Tinay. 1996. Changes in chemical composition of guava fruits during development and ripening. Food Chem. 59 (3), 395-399. [ Links ]
Calzavara, B.G., C.H. Müller, and O. Kahwage. 1984. Fruticultura tropical: O cupuçuzeiro, cultivo, beneficiamento e utilização do fruto. Embrapa; CPATU, Belém, Brazil. [ Links ]
Carvalho de, J.E.U., C.H. Müller, R.L. Benchimol, A.K. Kate, and R.M. Alves. 1999. Copoazú [Theobroma grandiflorum (Willd. Ex Spreng) Shum.]: Cultivo y utilización. Manual técnico. Tratado de Cooperación Amazónica (TCA). Secretaría Protempore Venezuela; FAO; Embrapa, Amazonía Oriental, Belem, Brazil. [ Links ]
Carrington, C.M.S and R.A.G. King. 2002. Fruit development and ripening in Barbados cherry, Malpighia emarginata DC. Sci. Hort. 92, 1-7. [ Links ]
Escobar, C.J., D. Criollo, and W. Herrera. 2009. Copoazú (Theobroma grandiflorum, Willd. Ex Spreng Schum) Variabilidad y manejo del cultivo en el piedemonte amazónico. Produmedios, Bogota. [ Links ]
Galvis, A. and M.S. Hernández. 1993. Análisis del crecimiento del fruto y determinación del momento de cosecha del arazá (Eugenia stipitata). Revista Colombia Amazónica 6(2), 107-121. [ Links ]
Garriz, P.I., H.L. Álvarez, and G.M. Colavita. 2005. Growth pattern of 'Abbé Fetel' pear fruits. Acta Hort. 674, 321-327. [ Links ]
Hernández, M.S. and A. Galvis. 1994. Análisis de crecimiento del fruto y determinación del momento de cosecha del Copoazú. Revista Colombia Amazónica 7(1-2), 157-167. [ Links ]
Hernández, M.S., J.A. Barrera, M.P. Carrillo, O. Martínez, L.M. Melgarejo, J.A. Galvis, A.E. Casas, and C. Bolaños. 2006. Crecimiento y desarrollo de los frutos de especies promisorias del género Theobroma, bajo condiciones de la Amazonía norte colombiana. pp. 107-136. In: Melgarejo, L.M., M.S. Hernández, J.A. Barrera, and M.P. Carrillo (eds.). 2006. Theobroma. Instituto Amazónico de Investigaciones Científicas (SINCHI); Universidad Nacional de Colombia; Ed. Scripto, Bogota. [ Links ]
Hernández, M.S., O. Martínez, and J.P. Fernández-Trujillo. 2007. Behavior of arazá (Eugenia stipitata Mc Vaugh) fruit quality traits during growth, development and ripening. Sci. Hort. 111, 220-227. [ Links ]
Hobson, G. 1999. Maduración del fruto. pp. 463-478. In: Azcón- Bieto, J. and M. Talón (eds.). Fisiología y bioquímica vegetal. 5th ed. Interamericana Mc-Graw-Hill, Madrid. [ Links ]
Ibarra-Manríquez, G. and G. Cornejo-Tenorio. 2010. Diversidad de frutos de los árboles del bosque tropical perennifolio de México. Acta Bot. Mex. 90, 51-104. [ Links ]
Kader, A.A. 2002a. Methods of gas mixing, sampling and analysis. pp. 145-148. In: Kader, A.A. (ed.) Postharvest technology of horticultural crops. Publ. 3311 University of California, Berkeley, CA. [ Links ]
Kader, A.A. 2002b. Postharvest biology and technology: An overview. pp. 39-47. In: Kader, A.A. (ed.) Postharvest technology of horticultural crops. Publ. 3311 University of California, Berkeley, CA. [ Links ]
Kays, S.J. and R.E. Paull. 2004. Postharvest Biology. Exon Press, Athens, GA. [ Links ]
López, A.F. and P.A. Gómez. 2000. Developing a ripening index for bell peppers based on color measurements. pp. 48-53. In: Artés, F., M.I. Gil, and M.A. Conesa (eds.). Improving postharvest technologies of fruits, vegetables and ornamentals. IIFR Congress. Vol. I. Murcia, Spain. [ Links ]
Mercado-Silva, E., P. Benito-Bautista, and M. García-Velasco de A. 1998. Fruit development, harvest index and ripening changes of guavas produced in central Mexico. Postharv. Biol. Technol. 13, 143-150. [ Links ]
Rodríguez, M., H.E. Arjona, and H.A. Campos. 2006. Caracterización fisicoquímica del crecimiento y desarrollo de los frutos de feijoa (Acca sellowiana Berg) en los clones 41 (Quimba) y 8-4. Agron. Colomb. 24(1), 54-61. [ Links ]
Rojas, S., J. Zapata, A. Pereira, E. Varón, C. Cardenas, and F. Cadena. 1998. El cultivo de Copoazú (Theobroma grandiflorum) en el piedemonte amazónico colombiano. 2nd ed. Corpoica; Fondo Amazónico, Florencia, Colombia. [ Links ]
Salisbury, F.B. and C.W. Ross. 2006. Fisiología de las plantas: desarrollo de las plantas y fisiología ambiental. Vol. 3. Thompson Editores Spain; Paraninfo, Madrid. [ Links ]
Seymour, G.B., J.E. Taylory, and G.A. Tucker. 1993. Biochemistry of fruit ripening. Chapman and Hall, London. [ Links ]
Statgraphics plus 5.1. 2000. Statistical graphics corp. STSC, Rockville, MA. [ Links ]
Sousa, M.S.B., L.M. Vieira, M.J.M. da Silva, and A. de Lima. 2011. Caracterização nutricional e compostos antioxidantes em resíduos de polpas de frutas tropicais. Ciênc. Agrotec. 35(3), 554-559. [ Links ]
Tadesse, T., E. Hewett, M.A. Nichols, and K.J. Fisher. 2002. Changes in physicochemical attributes of Sweet pepper cv. Domino during fruit growth and development. Sci. Hort. 93, 91-103. [ Links ]
Wills, R., B. Glasson, D. Graham, and D. Joy. 1998. Postharvest. An introduction to the physiology and handling of fruit, vegetables and ornamentals. 4th ed. CAB, Wallingford, UK. [ Links ]
Worrell, D.B., C.M.S. Carringtona, and D.J. Huber. 1998. Growth, maturation and ripening of breadfruit, Artocarpus altilis (Park.) Fosb. Sci. Hort. 76, 17-28. [ Links ]













