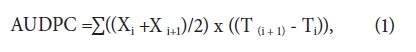Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Agronomía Colombiana
Print version ISSN 0120-9965
Agron. colomb. vol.30 no.2 Bogotá May/Aug. 2012
PLANT BREEDING, GENETIC RESOURCES & MOLECULAR BIOLOGY
Phenotypic evaluation of the resistance in F1 carnation populations to vascular wilt caused by Fusarium oxysporum f.sp. dianthi
Evaluación fenotípica de la resistencia a Fusarium oxysporum f.sp. dianthi en poblaciones F1 de clavel
Johana Carolina Soto-Sedano1,Myriam Judith Clavijo-Ortiz1 and Juan José Filgueira-Duarte1
1Faculty of Science, Universidad Militar Nueva Granada . Bogota (Colombia). juan.filgueira@unimilitar.edu.co Received for publication: 25 July, 2011. Accepted for publication: 31 August, 2012.ABSTRACT One of the most important phytosanitary problems of the carnation crops in Colombia and in the entire world is vascular wilting produced by Fusarium oxysporum f.sp. dianthi. Currently, an effective treatment against the pathogen does not exist; the search for resistant varieties has been the most successful method for control of this disease. Breeding programs are vital to solving the problem of the carnation fusariosis. The objective of this research was the phenotypic evaluation of carnation F1 populations, products of contrasting crossing, resistant per susceptible to F. oxysporum f.sp. dianthi, in order to determine if the resistance is inherited in the lines. This information will contribute to the selection of material and to the successful introduction of the resistant characteristic, whose expression is commercially acceptable, to the gene pool. The methodology adopted was a phenotypic evaluation of the response to the parasite in the population (450 individuals) and in the parental. This evaluation estimated the area under the curve (AU DPC), using a scale of symptoms reported for carnation vascular wilt. Three different phenotypes were established with this evaluation. The moderately susceptible one is the predominant phenotype and an analysis of phenotypic frequencies was carried out on it. The results show that the individuals of the evaluated F1 population were distributed between two extreme ranges, resistant and susceptible; this shows that there is segregation for the trait resistant to F. oxysporum f.sp dianthi. We did not observe clearly differentiated classes, i.e. with complete absence or presence of the disease, indicating a possible control of the resistance in the evaluated carnation material, governed by more than one gene and with a possible additive genetic action.
Key words: Dianthus caryophyllus, AU DPC, vascular wilt, polygenic.
RESUMEN Uno de los problemas fitosanitarios de mayor importancia para los cultivadores de clavel en Colombia y el mundo, es la marchitez vascular del clavel producida por Fusarium oxysporum f.sp. dianthi. En la actualidad no existen métodos eficaces para el control de este patógeno, sólo la búsqueda de variedades resistentes ha sido el método má s eficaz para el control de esta enfermedad. Los programas de fitomejoramiento tienen gran importancia en la solución del problema de la fusariosis en clavel. Así, el objetivo de esta investigación, fue evaluar fenotípicamente poblaciones F1 de clavel, producto entre cruces contrastantes, resistente por susceptible a F. oxysporum f.sp dianthi, con el fin de profundizar en el modo en que se hereda la resistencia en las líneas. Esta información contribuirá en la selección del material comercial y a la exitosa introducción del cará cter de resistencia dentro del pool de genes cuya expresión determine características comercialmente aceptadas. La metodología constó de una evaluación fenotípica de la respuesta al patógeno, de las poblaciones (450 individuos) así como de sus parentales. Esta evaluación se realizó estimando el á rea bajo la curva del progreso de la enfermedad (AU DPC), usando una escala de síntomas reportada para la marchitez vascular en clavel. Con esta evaluación, se establecieron tres fenotipos diferentes, siendo medianamente susceptible el predominante y se realizó un aná lisis de frecuencias fenotípicas. Los resultados muestran, que los individuos de las poblaciones F1 evaluadas, se distribuyen entre los dos rangos extremos, resistente y susceptible, así como en el rango intermedio de medianamente susceptible, mostrando que hay segregación para el rasgo de resistencia a F. oxysporum f.sp dianthi. No se presentó el caso de clases claramente diferenciable como ausencia o presencia total de la enfermedad, este hecho presume un posible control de la resistencia en el material de clavel evaluado, gobernado por má s de un gen y con una posible acción genética aditiva. Palabras clave: Dianthus caryophyllus, AU DPC, marchites vascular, poligénia.Introduction
Colombia has a great tradition in flower production and is the second largest exporter of cut fresh flowers in the world, after Holland, and the largest in the case of the carnation (Salinger, 1991; Asocolflores, 2011), thanks to the weather conditions and labor costs (Pizano, 1987). Of the total cultivated flower area in the country, 18% corresponds to the carnation; and the principal countries of importation of this flower are the United Stated, Japan and Holland (Asocolflores, 2011).
Carnation crops have been threatened for decades by the most limiting disease, vascular wilt, caused by Fusarium oxysporum f.sp. dianthi (Prill and Delacr) W.C. Snyder and H.N. Hansen. The loss caused by this disease may exceed 10% of total production. Chemical management is expensive and inefficient. The most successfully form to address the problem is the cultivation of resistant varieties, obtained by traditional breeding methods (Arbelá ez, 1987; Filgueira, 2009). So, the crop of resistant varieties in our country will produce an important reduction of production costs and avoid losses by the disease (Filgueira, 2011).
At the world level, Fusarium pathogens have increased in importance, putting flower-growers and growers in general on alert, because these pathogens have expanded their range of hosts and developed resistance to chemical treatment (Abeywickrawa and Beal, 1992).
Depending on the host that is attacked by F. oxysporum, the resistance of plants can be controlled by a simple gene (monogenic), by a few genes (oligogenic), or by multiple genes (multigenenic), (Berrocal-Lobo and Molina, 2007). In the particular case of a host affected by F. oxysporum, different types of inheritance have been described, monogenic and polygenic; and different resistant genes on different crops, including the tomato, melon, cucumber and kidney beans (Michielse and Rep, 2009).
Knowing that the resistant type depends on the vegetal material, the objective of this study was to determinate the form in which the resistance is inherited in lines of carnation, in our breeding program; in order to guide the adequate selection of proposed crosses to introduce genetic resistance to vascular wilt into the gene pool, whose expression determines accepted commercial characteristics. Likewise, this knowledge will contribute to future studies, which could address the identification and location of genes involved in resistance to F. oxysporum f.sp. dianthi in the carnation.
Knowledge of the genetics and inheritance of carnation resistance to F. oxysporum f.sp. dianthi will be a useful tool in the breeding program of the Molecular Phytopathology Group of Universidad Militar Nueva Granada (UMNG ). The principal target of the carnation breeding program is to obtain commercial plants resistant to Fusarium.
Material and methods
Biological material
We used three F1 populations of carnation, each one with 150 individuals, corresponding to contrasting crosses, between resistant parental plants (R) and susceptible plants (S) to F. oxysporum f.sp. dianthi. These population were: Kaly (S)(?) × Candy (R)(?), UM 503 (R)(?) × UM 226 (S) (?), and Candy (R)(?) × UM 226 (S)(?); representing the different possible combinations of the material in the carnation breeding programs of the UMNG .
The resistant and susceptible characteristics of the commercial carnation varieties Kaly and Candy and the lines UM 503 and UM 226 were used as parental lines and all of them were evaluated in greenhouse and laboratory conditions (Soto et al., 2009).
The F. oxysporum f.sp. dianthi inoculums that we used for the evaluation were obtained from commercial carnation plants; we used these inoculums to produce a monosporic culture, following the methodology described by Soto et al. (2009).
Parental response evaluation of the F1 population to vascular wilt
The response evaluation of the parental and F1 populations to F. oxysporum were observed simultaneously in all parental and for all the plants of the F1 populations. For the evaluation of the parental, we took 30 plants (clones) of each commercial variety and parental line. For the evaluation of the F1 population, we took three plants (clones) of each one. After 4 weeks, we inoculated them through immersion of the root for 30 s in a conidial suspension with a 1·106 conidia/ mL concentration, from the monosporic isolate of F.
oxysporum f.sp. dianthi. Once we inoculated the cuttings, the plants were planted in 8 oz plastic cups, in a substrate mix of sterile soil and rice hulls (3:1). For the negative control, 30 plants (clones) were taken of each parental line and three plants (clones), for each one of the F1 populations. These plants were submerged at the same time in sterile distilled water (SDW). Simultaneous to the F1 evaluation, a test was done to control infection and invasion by the pathogen. It consisted on the inoculation of 20 root cuttings of the susceptible Kaly commercial variety (with the same methodology and conditions). Each week, we took a plant at random; from this plant, we registered the internal and external symptoms and we reinsulated the pathogen. All the work was done under greenhouse conditions, keeping the sequence, tracking climate and temperature conditions and relative humidity. Irrigation was done using micro filter and UV treated water and fertilization was done using pre-established carnation management plans.
Characteristic vascular wilt symptoms were evaluated one week after the inoculation, every 7 d for 18 weeks, according to the following symptoms scale (modified by Cevallos et al., 1990; Arbelá ez et al., 1993; Benavides et al., 1995): 0, healthy plants without symptoms; 1, plants with symptoms of turgor loss; 2, plants with yellowing symptoms; 3, plants with severe wilting; 4, dead plants. When the time of symptom registration was finished, the pathogen was isolated from each inoculated plant to corroborate plant infection. With the registered symptoms and using the severity scale over the time of the experiment, the area under the disease progress curve (AU DPC) was calculated using this equation (1).
where i=1, Ti= No. days between inoculation and the sampling date, Xi = the rating using the symptoms scale, (T(i + 1) - Ti) = time in days between two readings, (Jeger y Viljanen-Rollinson, 2001). With the data for the AU DPC for each parental and each F1 plant, as well as with the detailed observation of the symptoms over time, the phenotypes were established in ranges using frequency analysis.
Finally, a variance analysis (ANOVA) was carried out between the AU DPC values obtained in the evaluation of F. oxysporum in the F1 population, with the purpose of establishing whether there were significant differences between them. Then, a genetic analysis of the resistance was performed; the normality distribution of the segregation characteristic was evaluated using the phenotypic frequencies and a Shapiro Wilks test of data normality.
Results and discussion
The infection and invasion of the positive control by the pathogen, showed that the infection and the invasion, occurred successfully after the inoculation, because the pathogen was isolated from plants of the Kaly variety which is the commercial, susceptible plant, corroborating the pathogenicity of the Fusarium used in the evaluation. In the evaluations of the response to vascular wilt in parental and F1 population, different categories of symptoms were identified that describe the fusariosis disease in the carnation (Cevallos et al., 1990; Arbelá ez et al., 1993; Benavides et al., 1995). The phenotype evaluation was based on the symptom scale and on the register of the biological response of the different materials to the pathogen, in order to establish possible phenotype ranges in the population.
Some plants of the population presented category 3 in the severity scale, surpassing the wilting level but they did not reach level 4, or dead. In this case, plants with unilateral wilt were observed. The wilt in other reports was attributed to a response of the plant to occlude and limit the advance of the pathogen with gels (Beckman and Halmos, 1962; Misaghi et al., 1978; Baayen, 1986).
Baayen (1986) showed that in moderately susceptible varieties of the carnation, there could be regeneration of the vascular tissue, after inoculation, infections and symptoms of wilt produced by F. oxysporum. Those events would be related to the resistant response in the Fusarium-carnation interaction.
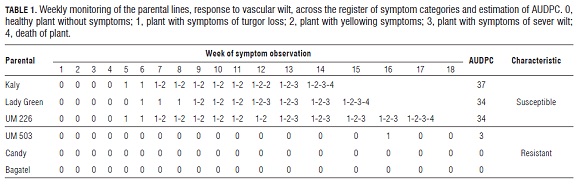
The results of the estimation of the AU DPC in the evaluation of the parental lines, showed values of the AU DPC between 34 and 37 for the resistant parental lines, with presented AU DPC values between 0 and 3 for the particular case of the UM 503 line (Tab.1). Meanwhile, the negative controls showed AU DPC values of zero, which present no symptoms related to the vascular wilt.
The symptoms began to be evident in the susceptible parental lines, starting with the 5th week of evaluation. The death of the plants was seen at the 14th week (Tab.1). The time in which the symptoms were present, both in parental plants and in the three evaluated populations, was variable, which shows that there is a varietal reply in the pathogen response. The time of evaluation of the symptomatology (18 week) and also the size of the three populations evaluated (150 plants each) allowed us to find a wide variety of situations, from plants that did not present any symptoms to plants that died from vascular wilt.
From the observation and detailed tracking of the symptomatology over time, for each plant of the three evaluated populations, the phenotype ranges were estimated as resistant (R), moderately susceptible (MS ) and susceptible (S). In the range R, plants that at the end of the symptom register time did not present any symptoms were placed. In the range MS , plants that presented symptoms in numbers one and two were placed. And finally, in the range S, the plants that presented symptoms in numbers 3 and 4 were placed, namely wilting and death. The AU DPC information of each plant of the different evaluated populations was used to support each phenotypic range (R, MS and S) for each population. These ranges describe the phenotypes of vascular wilt response that are evident in the populations across the symptomatology, and the time in which those symptoms are present.
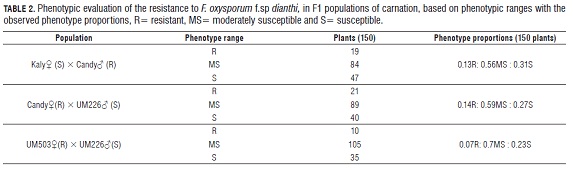
The results of the evaluations of vascular wilt response for the population from the Kaly? × Candy? cross, showed that the first symptoms were evident at the 2nd week of evaluation; while, week 8 was the average time in which the symptomatology in the susceptible plants was evident. Of 150 plants, 19 were in the range R, 47 in the range S, and the main proportion, 84 plants; were, in the range MS . The pattern of segregation in the population from the Kaly? × Candy? cross was 0.13R: 0.56MS : 0.31S (Tab.2).
According to the results, it was observed that the phenotype is distributed in the two extreme ranges, resistant and susceptible, and also in the intermediate range of moderately susceptible, showing that segregation for the resistance characteristic to F. oxysporum f.sp. dianthi exists. The frequency analysis of the AU DPC for the different populations produced three intervals which are based on the range of the set of values of AU DPC. The use of AU DPC has increased and it is recommended when the phenology or host growth does not determinate the increase of the disease progress (Jeger and Viljanen-Rollinson, 2001). Likewise, the estimation of AU DPC has different applications in evaluation of resistance levels in the field, and also in the evaluation of quantitative resistance in different pathosystems (Das et al., 1992; Singh, 1993; Birhman and Singh, 1995; Ma et al., 1995; Broers et al., 1996; Jeger and Viljanen-Rollinson, 2001), in particular for F. oxysporum investigations on watermelon (Perchepied and Pitrat, 2004; Navas et al., 2000) and the carnation (Cooke, 2006; Ben- Yephet, 1996).
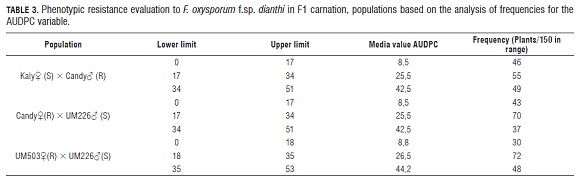
Table 3 shows the AU DPC behavior for the population from the Kaly? × Candy? cross. In this case, of 150 plants, 46 were in the low interval with a value of 8.5, 55 plants were placed in the middle interval with a value of 25.5 and 40 plants had a media value for AU DPC of 42.5 (Tab.3). The distribution of the plants in the media value intervals for AU DPC, presented a high similarity with the results obtained in the evaluation of the response to vascular wilt, based on the phenotypic ranges. In both cases, the main proportion of the plants was placed in the intervals of AU DPC with a media value of 42.5 and (MS ) moderate susceptible plants for the evaluation of phenotype ranges. The rest of the plants were at the end intervals, showing that the distribution of the phenotype response to vascular wilt, presents a tendency toward a normal distribution for the population from the Kaly? × Candy? cross, these results made a curve with a media value in the peak and values in the ends.
Likewise, in that population, it was observed that some plants present a more susceptible phenotype compared with the susceptible parental, estimated across the AU DPC. The susceptible parental was Kaly with an AU DPC of 37, of the 150 plants that were evaluated, 28 presented a higher AU DPC, the highest value was 51.
In the population of plants obtained from the cross Candy? × UM 226?, the first symptoms were presented in the 4th week of evaluation. The 8th week was the average time in which the symptomatology in the susceptible plants was evident, this characteristic was presented in a similar way in the population from the cross Kaly? × Candy?. From this population, of 150 plants, 21 plants were in the range R, 40 plants in the range S and 89 plants with the highest proportion in the range MS . The pattern of segregation for this population was, 0.14 R: 0.59 MS : 0.27 S (Tab.2). In a similar way as the population from the cross Kaly? × Candy?, the population form the cross Candy? × UM 226? had plants that were distributed in the intervals resistant and susceptible and also in the middle interval MS . This distribution also showed segregation for the characteristic of resistance to Fusarium.
The AU DPC analysis on the progeny from the cross Candy? × UM 226? gave the following results: from this population, 43 plants were in the lower interval with a media value of 8.5, 70 plants were in the middle interval with a media value of 25.5 and finally 37 plants were in the interval with a media value of 42.5 (Tab.3). The similitude between the normal distribution for the AU DPC of the plants in the population and the result of the evaluation of the response to vascular wilt based on the phenotypic ranges is also conserved for the population from the cross Candy? × UM 226?. Likewise, in this population, we also observed that some of the plants showed a phenotype more susceptible compared with the susceptible parental, which in this case was UM 226; this was measured thought the AU DPC.
The media value for the AU DPC of the susceptible parental UM 226 was 34 and this value was also the upper limit in the evaluation of the progeny and it correlated with the MS phenotype and also the lower limit of this study correlated with phenotype S. Likewise, in the evaluation of the plants progeny, there were higher values of AU DPC in 39 plants of 150 in the population, with a maximum AU DPC of 51. Finally, in the population from the cross UM 503? × UM 226?, the first symptom presented in the 3rd week of evaluation, and week 6 was the average week for the onset of symptoms; therefore, this cross presented symptoms earlier. Of the 150 plants of this population, 10 plants were in the range R, 35 plants in the range S, and 105 plants in the range MS ; the segregation pattern of the population from the UM 503? × UM 226? cross was 0.07R:0.7MS :0.23S. Of the three populations, this last one presented the highest number of plants with the phenotype MS (Tab.2).
The population from the UM 503? × UM 226 cross presented a distribution of the plants in the ranges R and S and a higher part of the population was placed in the MS range in the same way as the populations from the Kaly? × Candy? cross and the Candy? × UM 226 cross. This showed again that there is segregation for the resistance characteristic to F. oxysporum f.sp. dianthi. The analysis of the AU DPC on the progeny from the UM 503? × UM 226 cross, showed that the amplitude of the three intervals had a value of 18, the lower value for AU DPC was 0 and the highest was 53. For this population, 30 plants were in the lower interval, with a media value of 8.8, 72 plants where in the middle interval with a media value of 22.5, and 48 plants were in the interval with a media value of 44.2 (Tab.3).
The similitude between the normal distribution of the plants in the intervals obtained from the media values for AU DPC, and the result of the evaluation of vascular wilt response, based on phenotypic ranges, was again conserved for the cross UM 503? × UM 226?. Likewise, in this population, it was observed that some plants showed a more susceptible phenotype, compared with the susceptible parental, in the UM 226 case. The value of AU DPC of the susceptible parental UM 226, was 34 and in the evaluation of the progeny, some plants presented values of AU DPC higher than 49 with a maximum AU DPC value of 53.
The population from the UM 503? × UM 226? cross showed that some of the plants had a AU DPC value lower compared with the resistant parental, in this case UM 503, with a AU DPC value of 3; therefore, there were plants in the progeny, with a more resistant phenotype than the resistant parental.
None of the control plants showed a symptom related with vascular wilt, and the isolation of the pathogen from all the inoculated material, including the three repetitions, controls and parental lines at the end of the register time, corroborated the entry of the pathogen into the plants that were symptomatic.
The analysis of variance, established significant differences in the AU DPC variable, between the different individuals in each population and also that, between the three F1 populations, significant differences does not exist. This suggests that although the parental origin is not the same, since Kaly and Candy are standard commercial type varieties and UM 226 and UM 503 are lines produced by the cross between commercial parental, the obtained populations share the same response tendency, to F. oxysporum f.sp. dianthi.
The distribution of the segregation of the characteristic in the phenotype frequencies for the three populations presented a normal distribution tendency, as well as, the tendency of the AU DPC in the Shapiro Wilks test, which was significant, with values of 0.92 for the population from the Kaly? × Candy? cross; 0.76 for the Candy? × UM 226? cross and 0.91 for the UM 503? × UM 226? cross.
An important characteristic seen in the results of the evaluation of the F1 populations was that part of the progeny is out of the AU DPC range of the parents. This characteristic is known as transgressive segregation in the literature. In this case, plants of the F1 populations presented AU DPC values higher than the susceptible progenitor (Tab.1 and 3). It is known that when the inheritance of a trait is governed in a qualitative way, the phenotypes of the individuals are easily distinguishable in two different classes. For the case of resistance, they would be resistant and susceptible. However, characteristics that are controlled by multiples genes cannot be classified into simple classes (Agrios, 2005; Brown and Caligari, 2007).
The genetics of the inheritance of resistance in the carnation have been little explored. There are only some published studies that had the intention to deepen the knowledge of the genetics of this pathosystem. The first of them corresponds to the work of Sparnaaij and Demmink (1977), they proposed that resistance to F. oxysporum f.sp. dianthi raze 2 is polygenic and additive.
In addition, Bayeen et al. (1966) and Scovel et al. (2001), worked with populations, produced by the cross of different populations, they proposed that the inheritance of resistance in the carnation is additive and at least two genes exist.
An investigation developed in Spain for carnation crops evaluated populations, generated by crossing susceptible and resistant varieties, proposed that resistance in the carnation is produced by several genes but is only expressed when all of them are heterozygote for the allele that produces the resistance, and susceptibility is determined by the presence of one or more homozygote recessive loci (Arus et al., 1992).
The carnation is a highly heterozygote species, due to its condition as an allogamus plant, due to its long history of breeding and selection (Baayen et al., 1991). However, segregation analysis in the carnation, as we present in this study, should be developed by crossing or autopollination between commercial varieties. On the other hand, it is known that the carnation genome is diploid, with a chromosomal formulate of 2n=30 (Gatt et al., 1998; Castilla et al., 2009), meaning that the number of diploid allelic combinations in the crosses would be three.
Finally, the present study established three different phenotypes but the predominant phenotype was (MS ) moderately susceptible. Neither complete absence nor complete presence of the disease was observed for the range or intervals. These facts presume a possible control of resistance in carnation plants which is governed by more than two genes and with a possible additive genetic action.
Acknowledgment
Thanks to the Universidad Militar "Nueva Granada" and the "Ministerio de Agricultura y Desarrollo Rural" of Colombia for financial support through the CIAS -302 project and contract UMNG -MA DR 008-200887271-3244.
Literature cited
Abeywickrawa, A. and S. Beal. 1992. The Fusarium relation races. Physiol. Mol. Plant Pathol. 32, 89-92. [ Links ]
Agrios, G. 2005. Plant pathology. 5th ed. Elsevier Academic Press, San Diego, CA. pp. 952-953. [ Links ]
Arbelá ez, G., O. Calderón, F. Cevallos, and D. Gonzá lez. 1993. Determinación de las razas fisiológicas de Fusarium oxysporum f.sp. dianthi en clavel en la Sabana de Bogotá . Agron. Colomb. 10, 19-32. [ Links ]
Arbelá ez, G. 1987. Fungal and bacterial disease on carnation in Colombia. Acta Hort. 216, 151-157. [ Links ]
Arus, P., M. Llaurado, and J. Pera. 1992. Progeny analysis of crosses between phenotypes resistant and susceptible to Fusarium oxysporum f.sp. dianthi race 2. Acta Hort. 307, 57-64. [ Links ]
Asocolflores, Colombian Association of Flower Exporters. 2011. Floriculture for export. In: http://www.asocolflores.org; consulted: March, 2012. [ Links ]
Baayen, R. L. Sparnaaij, J. Jansen, and G. Niemann. 1991. Inheritance of resistance in carnation against Fusarium oxysporum f.sp. dianthi races 1 and 2, relation to resistance components. Neth. J. Plant Pathol. 97, 73-86. [ Links ]
Baayen, R. 1986. Regeneration of vascular tissues in relation to Fusarium wilts resistance of Carnation. Neth. J. Plant Pathol. 92, 273-285. [ Links ]
Beckman, C. and S. Halmos. 1962. Relation of vascular occluding reactions in banana roots to pathogenicity of root-invading fungi. Phytopathology 52, 893-897. [ Links ]
Benavides, J., E. Garcés, G. Arbelá ez, and F. Dukuara. 1995. Determinación de razas fisiológicas de Fusarium oxysporum f.sp. dianthi en suelos cultivados y en variedades de clavel en la finca "Flores las Palmas". Agron. Colomb. 12(1), 21-26. [ Links ]
Ben-Yephet, Y., M. Reuven, A. Zveibil, and D. Shtienberg. 1996. Effects of abiotic variables on the response of carnation cultivars to Fusarium oxysporum f.sp. dianthi. Plant Pathol. 45(1), 98-105. [ Links ]
Berrocal-Lobo M. and A. Molina. 2007. Arabidopsis defense response against Fusarium oxysporum. Trends Plant Sci. 13(3), 145-150. [ Links ]
Birhman R. and B. Singh. 1995. Path-coefficient analyses and genetic parameters of the components of field resistance of potatoes to late blight. Ann. Appl. Biol. 127, 353-362. [ Links ]
Broers, L. X. Cuesta, and R. Lopez. 1996. Field assessment of quantitative resistance to yellow rust in ten spring bread wheat cultivars. Euphytica 90, 9-16. [ Links ]
Brown, J. and P. Caligari. 2007. An introduction to plant breeding. Blackwell Publishing, Ames, IA . pp. 209-212. [ Links ]
Castilla, Y., M.E. Gonzá lez, X. Xiques, and A. Pinares. 2009. Establecimiento de una metodología para el conteo de cromosomas en el clavel español (Dianthus caryophyllus L.). Rev. Cultivos Trop. 30(1), 65-68. [ Links ]
Cevallos, J., D. Gonzá lez, and G. Arbelá ez. 1990. Determinación de razas fisiológicas de Fusarium oxysporum f.sp. dianthi en clavel en la Sabana de Bogotá . Agron. Colomb. 7, 40-46. [ Links ]
Cooke, B., D. Gareth, and B. Kaye. 2006. The epidemiology of plant diseases. 2nd ed. Springer-Verlag, New York, NY . pp. 600-602. [ Links ]
Das, M., S. Rajaram, C. Mundt, and W. Kronstad. 1992. Inheritance of slow-rusting resistance to leaf rust in wheat. Crop Sci. 32, 1452-1456. [ Links ]
Filgueira, J. 2011. Experiencias en mejoramiento del clavel (Diantus caryophyllus). Universidad Militar Nueva Granada, Bogota. pp. 183-185. [ Links ]
Filgueira, J. 2009. Estudio y manejo de la pudrición basal producida por hongos del complejo Fusarium en clavel comercial en la Sabana de Bogotá . Revista de la Asociación Colombiana de Exportadores de Flores (Asocolflores) 72, 53-54. [ Links ]
Gatt, M., K. Hammett, K. Markham, and B. Murray. 1998. Yellow pinks: interspecific hybridization between Dianthus plumarius and related species with yellow flowers. Scientia Hort. 77(3), 207-218. [ Links ]
Jeger, M. and S. Viljanen-Rollinson. 2001. The use of the area under the disease-progress curve (AU DPC) to assess quantitative disease resistance in crop cultivars. Theor. Appl. Genet. 102, 32-40. [ Links ]
Ma, H. and A. Mujeeb-Kazi. 1995. Resistance to stripe rust in Triticum turgidum, T. tauschii, and their synthetic hexaploids. Euphytica 82, 117-124. [ Links ]
Michielse, B. and M. Rep. 2009. Pathogen profile. pathogen profile update: Fusarium oxysporum. Mol. Plant Pathol. 10(3), 311-324. [ Links ]
Misaghi, I., J. De Vay, and J. Duniway. 1978. Relationship between occlusion of xylem elements and disease symptoms in leaves of cotton plants infected with Verticillium dahliae. Can. J. Bot. 56, 339-342. [ Links ]
Navas, J. B. Hau, and R. Jiménez. 1998. Effect of sowing date, host cultivar, and race of Fusarium oxysporum f.sp. ciceris on development of Fusarium wilt of chickpea. Phytopathology 88, 1338-1346. [ Links ]
Perchepied, L. and M. Pitrat. 2004. Polygenic inheritance of partial resistance to Fusarium oxysporum f.sp. melonis race 1.2 in melon. Phytopathology 94, 1331-1336. [ Links ]
Pizano, M. 1987. Carnation culture in Colombia: State of the art. Acta Hort. 1, 5-6. [ Links ]
Salinger, J. 1991. El clavel. Producción comercial de flores. Editorial Acribia, Zaragoza, Spain. [ Links ]
Scovel, G. M. Ovadis, and A. Vainstein. 2001. Market assisted selection for resistance to Fusarium oxysporum in the greenhouse carnation. Acta Hort. 552, 151-156. [ Links ]
Singh, R. 1993. Resistance to leaf rust in 26 Mexican wheat cultivars. Crop Sci. 33, 633-637. [ Links ]
Shaner, G. and R. Finney. 1977. The effect of nitrogen fertilization on the expression of slow-mildewing resistance in Knox wheat. Phytopathology 67, 1051-1056. [ Links ]
Sparnaaij, l. and J. Demmink. 1977. Progress towards Fusarium resistance in carnations. Acta Hort. 71, 107-113. [ Links ]
Soto, J., F. Pabón, and J. Filgueira. 2009. Relación entre el color de la flor del clavel (Dianthus caryophyllus) y la tolerancia a patógenos del género Fusarium. Rev. Fac. Cienc. Bá sicas 5, 116-129. [ Links ]