Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Agronomía Colombiana
versão impressa ISSN 0120-9965
Agron. colomb. vol.30 no.2 Bogotá maio/ago. 2012
POSTHARVEST PHYSIOLOGY & TECHNOLOGY
Lipophilic antioxidant activity of guava fruit varieties Palmira ICA I, Regional Roja and Regional Blanca in four ripening stages
Actividad antioxidante lipofílica de guayaba variedades Palmira ICA I, Regional Roja y Regional Blanca en cuatro estados de maduración
Mauricio Espinal R.1 , Jorge Iván Daza A.1 and Luz Patricia Restrepo S. 1
1Department of Chemistry, Faculty of Sciences, Universidad Nacional de Colombia. Bogota (Colombia). lprestrepos@unal.edu.co Received for publication: 15 June, 2010. Accepted for publication: 29 June, 2012.ABSTRACT
We determined the lipophilic antioxidant activity and β-carotene content with HPLC-UV in guava fruit Psidium guajava L.) varieties Palmira ICA I, Regional Roja and Regional Blanca in the ripening stages: green, semi-ripe, mature and senescent. It was established that the β-carotene content and lipophilic antioxidant activity increased during the ripening process up to the climacteric maximum and decreased during senescence; lipophilic antioxidant activity being higher in the varieties Palmira ICA I (13.06 μmol α-tocoferol/g fruit) and regional roja (14.08 μmol a-tocopherol/g fruit) and lower in the regional blanca variety (7.04 μmol a-tocopherol/g fruit), while β-carotene content was highest in the regional roja variety (85.26 Eq. retinol/100 g fruit) followed by the varieties Palmira ICA I (10.53 Eq. retinol/100 g fruit) and regional blanca (5.78 Eq. retinol/100 g fruit). The best correlation between lipophilic antioxidant activity and β-carotene content was observed in the 'regional roja' (r2 = 0.830), while the varieties Palmira ICA I and regional blanca showed no correlation.
Key words: Psidium guajava L., β-carotene, postharvest, regional varieties.
RESUMEN
Se determinó la actividad antioxidante lipofílica y el contenido de β-caroteno por HPLC-UV en frutos de guayaba (Psidium guajava L.) variedades Palmira ICA I, regional roja y regional blanca en los estados de maduración verde, pintón, maduro y senescente. Se estableció que el contenido de β-caroteno y la actividad antioxidante lipofílica aumentaron durante el proceso de maduración hasta el máximo climatérico y disminuyó durante la etapa de senescencia, siendo la actividad antioxidante lipofílica mayor en las variedades Palmira ICA I (13,06 μmol α-tocoferol/g fruta) y regional roja (14,08 μmol α-tocoferol/g fruta) y menor en la variedad regional blanca (7,04 μmol α-tocoferol / g fruta), mientras que el contenido de β-caroteno fue mayor en la variedad regional roja (85,26 Eq. retinol/100 g fruta), seguido de las variedades Palmira ICA I (10,53 Eq. retinol/100 g fruta) y regional blanca (5,78 Eq. retinol/100 g fruta). La mejor correlación obtenida entre la actividad antioxidante lipofílica y el contenido de β-caroteno fue observada en 'regional roja' (r2 = 0,830), mientras que en las variedades Palmira ICA I y regional blanca no se observó ninguna correlación.
Palabras clave: Psidium guajava L., β-caroteno, poscosecha, vaiedades regionales.
Introduction
Guava (Psidium guajava L.) is a fruit from countries located in the tropics and subtropics and is attributed with a large number of functional properties such as antioxidant, antidiarrheal, anti-malarial, anti-cancer, anti-inflammatory and others (Pérez-Gutiérrez et al., 2008). Guava is a climacteric fruit, having high levels of ascorbic acid, polyphenolics, carotenoids, pectin, carbohydrates and some minerals (El-Bulk et al., 1997). It is well known that the guava fruit contains a very high antioxidant potential and has a great ability to prevent diseases aβociated with oxidative stress. β-carotene is the most common carotenoid with lipophilic antioxidant activity and pro-vitamin A activity (Alquezar et al., 2008). The lipophilic antioxidant activity of β-carotene has been associated with the ability to induce protection against ionizing radiation, a singlet oxygen neutralizing capacity and a peroxyl radicals neutralization capacity (Miller et al., 1996).
The benefits that have been associated with vitamin A include: intervention in the processes of bone and soft tissue and mucous membrane formation, antioxidant activity, pigment generation necessary for the functioning of the retina such as retinoic acid, reduction of the risk of chronic diseases such as cardiovascular diseases, cancer and diabetes and participation in the processes of reproduction and lactation (Burri, 1997). This research aimed to study the behavior of β-carotene during the maturation process and its relation to the lipophilic antioxidant activity of the guava fruit varieties Palmira ICA I, regional roja and regional blanca.
Materials and methods
Plant material
Guava fruit varieties Palmira ICA I, regional roja and regional blanca were used in the ripening states: green, semi-ripe, mature and senescent. These stages of maturity were initially selected for their color; where the green stage has a 100% green color, the semi-ripe stage has a 50% green and 50% yellow color, the mature stage has a 100% yellow color and the senescent stage has a 100% ocher color, characterized by enzymatic browning. The fruits of the Palmira ICA I variety were purchased in the town of Puente Nacional (1,625 m a.s.l. average temperature of 19°C) and the varieties regional roja and regional blanca were acquired in the municipality of Barbosa (1,610 m a.s.l. and average temperature of 21°C), located in the Velez province of the Santander department, Colombia. All fruits were collected in the month of January (precipitation of 137.6 mm/month).
Physicochemical characterization of the maturity stages
In order to characterize the maturity stage of the fruits used, the following determinations were made: Respiratory intensity: Respiratory intensity was measured according to the amount of O2 consumed by a pre-weighed fruit (n = 9) for 1 h during storage in a 1 L hermetic chamber. The translocation was carried out during the day with an average temperature of 19°C. Measurement of O2 uptake was performed using a selective electrochemical O2 sensor O2-BTA Vernier of 0 to 27 ppt coupled to a Texas Instrument TI 83 Plus calculator. The results were expressed as mg O2/kg fruit per hour.Maturity index: The maturity index was calculated as the ratio of soluble solids and titratable acidity (°Brix /% citric acid). Soluble solids were measured using a Atago 8382 manual sucrose refractometer and expressed as °Brix at a temperature of 18°C. Titratable acidity was measured by titration with a standard solution of 0.05 M sodium hydroxide with about 1 g of sample until the phenolphthalein endpoint. The titratable acidity was expressed as %w/w of citric acid because it constitutes the majority of organic acid of guava fruits (Wilson et al., 1982).
Color: The color measurement was taken using a Minolta CR-300 reflectance colorimeter with a C light source. Nine replications were performed on three fruits of the same maturity stage (n = 27). The color measurement was taken in the epicarp and the mesocarp of the fruit. In the epicarp, the measurement was taken directly on the fruit surface whereas in the mesocarp, the color measurement was done using a cut parallel to the equatorial axis of the fruit, avoiding the seeds. The results were expressed with the Color Index for Red Grapes (CIRG), defined as CIRG = (180 - h *) / (L * + C *), where h * corresponds to the hue, L * to luminosity and C * to chroma value (Carreño et al., 1995). Firmness: The compression force profiles were measured with a TA XT PLUS texturometer (Stable Micro Systems, New York, NY ). There were nine replications with three fruits of the same maturity stage (n = 27). We used a stainless steel probe P/2 of 2 mm diameter. The piston speed before the compression test was 1 mm/s, during the test, it was 0.2 mm/s, and after the test, it was 10 mm/s. The piston penetration distance was 15 mm with a trigger force of 100 g. The compression force for the epicarp and the mesocarp was expressed as kilograms force (kgf).
Determination of b-carotene with HPLC -UV
Extraction of β-carotene was performed with the procedure established by Bueno (1997). Approximately 5 g of fruit (edible part of the fruit excluding seeds) was added to 10 mL of acetone with 1% BHT and agitated for 24 h at 4°C under dark conditions and centrifuged at 5,550 gn and 4°C for 15 min. The supernatant was filtered with a 0.45 μm Target® PVDF filter and injected into the chromatograph. The finetuning of the RP-HPLC-UV methodology for the detection and quantification of β-carotene was performed with the procedure established by Gartner and Restrepo (1997). The parameters were determination of the linearity interval, correlation coefficient and confidence limits, sensitivity (limit of detection and quantification), precision (coefficient of variation and confidence limits) and accuracy (relative error and percentage recovery ). The calibration curve was performed in triplicate (n = 3), while for the determination of accuracy and precision of the standard and the sample, five injections were performed (n = 5). We used a Shimadzu LC-20AT high efficiency liquid chromatograph with a UV / VIS SPD-20A detector. The chromatographic method used was a reversed-phase method with the following chromatographic conditions: RP-18 column of 150 mm x 4 mm x 5 μm, isocratic elution with a mobile phase of acetonitriledichloromethane 75:25% v/v with 1% BHT, flow of 0.5 mL min-1, UV / VIS detector at 450 nm, injection loop of 20 uL and run time of 13 min (Xu et al., 2006). The calibration curve was performed using a 95% I SIGMA type b-carotene chromatographic pattern at concentrations between 0.24 and 60.56 equivalents of retinol mL (Eq. retinol/mL). β-carotene was expressed as equivalents of retinol/100 g of fruit (Eq. retinol/100 g fruit). The measurements of the samples were performed in triplicate in three fruits of the same maturity stage (n = 9).
Measurement of lipophilic antioxidant activity
Lipophilic antioxidant activity was determined with the β-carotene bleaching method (Velioglu et al., 1998). Approximately 0.2 g of sample (edible part of the fruit of guava excluding seeds) was added to 4 mL of an extractant solution (HCl-methanol-water 80:19:1%) and agitated at room temperature at 200 rpm in a vortex for 120 min and centrifuged at 5,550 gn and 4°C for 10 min. The supernatant was stored at -20°C under dark conditions during the analysis, for which 500 mL of a b-carotene solution was taken in chloroform at 0.4 mg mL-1 and the chloroform was evaporated at room temperature. 20 mL of linoleic acid, 200 m L of Tween®-20 and 200 mL of an antioxidant compounds extract were added, followed by 50 mL of deionized water saturated with oxygen by bubbling air, and it was incubated at 50°C for 2 h. Absorbance was read at 470 nm for 2 h every 10 min. The sample (extract), a target (extraction solution) and a standard of a-tocopherol 50 μg mL-1 were measured. The lipophilic antioxidant activity was expressed as μg a-tocopherol/g fruit. The measurements were performed in triplicate on three samples of fruit with the same maturity stage (n = 9).
Data analysis
The average was used as a measure of centralization and standard deviation was used as a measure of dispersion. To determine significant differences between the measured response variables for each of the maturity stages and for each of the analyzed guava varieties, we performed an analysis of variance (AN OVA) of two factors with multiple samples per group including a Fisher LSD multiple comparison test (Least Significant Difference) with a significance level of P≤0.05
Results and discussion
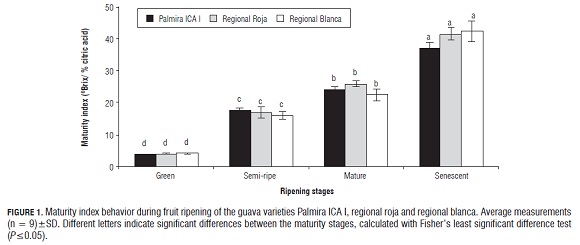
Physicochemical characterization of the maturity stages The physicochemical characterization of the maturity stages of the guava fruits was performed as a control of the maturation process, in order to ensure that the fruits did not suffer metabolic disorders associated with physical damage and that the lipophilic antioxidant activity and the β-carotene concentration during the maturation process corresponded to changes of metabolic alterations of normal fruit development and ripening and were not generated by responses to stress associated with metabolic alterations due to physiological damage (Nerd and Mizrahi, 1999). The parameter most commonly used as a control of the ripening process in fruit is the maturity index. It was found (Fig.1) that with increases in the maturity stage of the guava fruit varieties Palmira ICA, regional roja and regional blanca, from the green stage through semi-ripe and ripe stages, until the senescent stage, the maturity index increases progressively, allowing the maturity index to be used as a Physicochemical parameter control of the fruit maturity stage. The maturity index will be used hereafter to characterize the maturity stage of the three guava fruit varieties. Due to the progressive increase in soluble solids, resulting from the hydrolytic degradation of carbohydrate polymers such as pectin and starch and the appearance of soluble monosaccharides such as glucose and galacturonic acid and the titratable acidity reduction caused by the metabolism of organic acids, the maturity index can be used as a physicochemical parameter control of the maturity stage of the guava fruit since both soluble solids and titratable acidity are chemical parameters characteristic of the normal metabolism of the fruits, because they represent the metabolic processes of the major substrates involved in the process of obtaining energy from the fruit during maturation (Saradhuldhat and Paull, 2007). This behavior of the maturity index in guava fruit has been reported by El-Bulk et al. (1997), Mercado-Silva et al. (1998) and Bashir et al. (2003), and other fruits such as melon (Villanueva et al., 2004), pitaya (Nerd and Mizrahi, 1999), banana (Palomar et al., 2005), pineapple (Saradhuldhat and Paull, 2007) and mango (Montalvo et al., 2007).
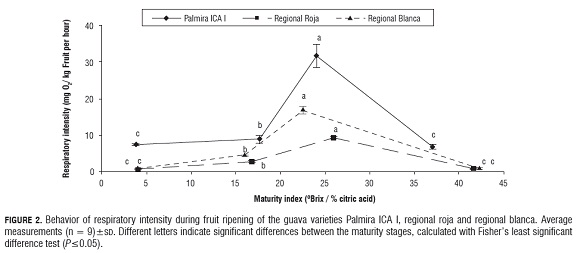
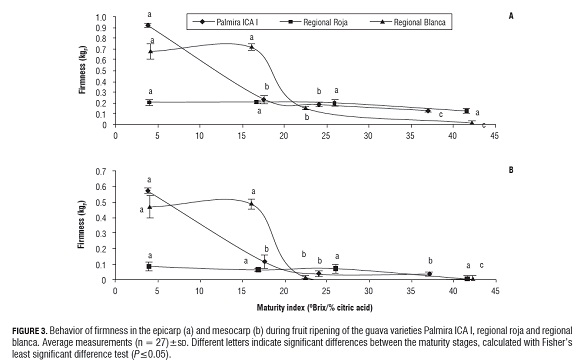
Since the climacteric respiratory pattern of the guava fruit is well known (Bashir et al., 2003), this physicochemical parameter can also be used as a suitable process control of fruit ripening. In guava fruits of the three varieties (Fig.2), it was found that with the increasing maturity of the fruit, the respiratory rate of the three varieties of guava increased to the climacteric maximum in the maturity stage of the fruit, where the best sensory characteristics are found, and decreased during the senescent phase of the fruits. It was observed that the guava variety Palmira ICA I showed the highest respiration intensity throughout the maturation process, followed by regional roja, while the lowest respiration intensity was in regional blanca. The relationship between respiration intensity and fruit ripening and senescence has been widely studied by El- Buluk et al. (1995), Mercado-Silva et al. (1998), Bashir et al. (2003), Bassetto et al. (2005) and Singh and Pal (2008), while climacteric behavior in other fruits has been found to be: in mango 19.73 mg O2/kg fruit per hour (Ravindra and Goswami, 2008), tomato (21 mg O2/kg fruit per hour), apple (28.4 mg O2/kg fruit per hour), strawberry (18.0 mg O2/kg fruit per hour) and blackberry (21.0 mg O2/kg fruit per hour) (Fonseca et al., 2002).Because the metabolic processes associated with the degradation of carbohydrates responsible for the structural conformation of the cellular wall such as pectic substances, starch and cellulose, and the appearance of soluble carbohydrates, the fruits suffer enzymatic softening during ripening (Ali et al., 2004). Fig.3 shows that as the guava fruit matures, both the firmness of the epicarp and of the mesocarp decrease; the decrease being higher in mesocarp firmness than in the epicarp, probably due to its high content of pectin, starch, cellulose and other polysaccharides responsible for the structural conformation of the cell wall (Duan et al., 2008). In the epicarp of the guava fruit variety regional roja, a significant change in firmness during the ripening process was not seen, being the least firm variety. In the epicarp of the guava variety regional blanca, we observed no significant difference in the firmness of the green or semi-ripe stages, while the mature and senescent stages showed a significant decrease in firmness, while the variety Palmira ICA I presented a significant decrease between the green stage and the semi-ripe stage, and remained constant for the mature and senescent stages (Fig.3 a), suggesting that the guava variety Palmira ICA I is more resistant to softening than the regional roja and regional blanca varieties.
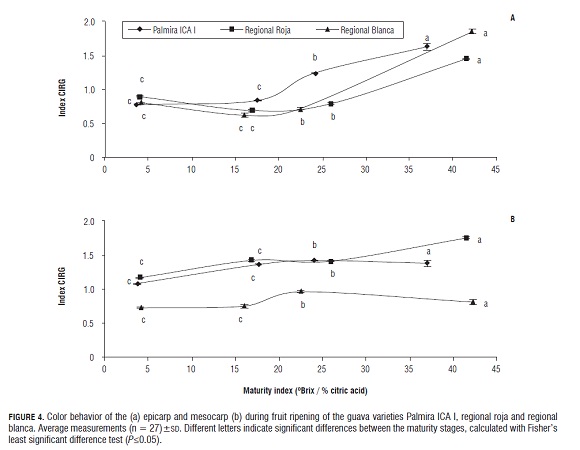
In Fig.3 b, one can see that the behavior of mesocarp firmness of the fruit of the three guava varieties behaved in the same way as in the epicarp, but that values are lower in mesocarp firmness than in the epicarp. Since there was no anomalous behavior in the process of fruit softening, we can say that the studied guava fruits did not have any metabolic disorders and had a process of maturation and senescence characteristic of the guava fruit (Muruyama et al., 1998).Chemical and biochemical changes occurring during the fruit ripening process can be evidenced visually by the color change in the epicarp and the mesocarp. The color changes of the fruits may be used as one indicative factor of the normal maturation process of the guava fruit, and allows for the correlation of color change with the appearance of functional characteristics such as antioxidant activity due to the appearance of carotenoids and anthocyanins. Fig.4 shows that the index CIRG progressively increases during the maturation process in the three varieties of guava, where the color change in the epicarp (Fig.4 a) is more significant than the color change in the mesocarp (Fig.4 b ).
It can be seen that in the mesocarp of the guava fruit varieties Palmira ICA I and regional roja, color changes are more significant than the color change that occurs in the variety regional blanca while the color change occurring in the epicarp of the variety Palmira ICA I is greater than the color change which occurs in the regional blanca variety, followed by regional roja. In varieties Palmira ICA I and regional roja, it was observed that the epicarp of the fruit was green in the green maturity stage, changing to yellow in the semi-ripe and mature stages, turning brown in the senescent stage, which is a color characteristic of the appearance of melanin due to the enzymatic browning process (Quevedo et al., 2009), while the mesocarp was red in all maturity stages, only increasing in intensity during the ripening process. The epicarp of the regional blanca variety was green in the green maturity stage, changing to yellow in the semi-ripe and mature stages, turning brown in the senescent stage, while its mesocarp was white throughout the process; of maturation, indicating a low carotenoid content in this strain. Because there were no strange colors characteristic of physiological damage and there was only damage due to senescence in the fruits of the three varieties, it can be suggested that all the color changes that occurred in the fruit were due to the fruit maturation process and none showed the presence of metabolic disorders such as the appearance of necrotic spots.Quantification of β-carotene and lipophilic antioxidant activity
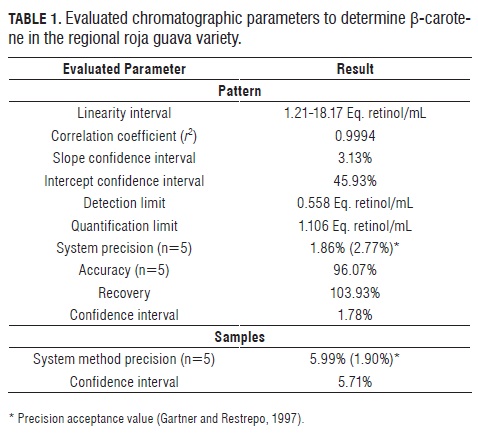
Analytical methodology was established with HPLC-UV for reliable quantification of β-carotene in guava samples of the varieties Palmira ICA I, regional roja and regional blanca. Tab.1 shows the chromatographic parameters evaluated for the development of the analytical methodology for quantification of β-carotene in the red guava variety because this variety has the highest concentrations of b-carotene.
At the upper limit of the linear interval of the curve (18.36 Eq. retinol/mL), precision, accuracy, the recovery rate and the confidence intervals of the chromatographic system were established as well as the precision and confidence intervals of the extraction method of β-carotene for the regional roja variety because it has the highest contents of b-carotene.With the development of the analytical methodology, it can be established that the linearity interval of the method is between 1.21 and 18.17 Eq. retinol/mL with a Pearson correlation coefficient (r2 = 0.9994), the confidence intervals were found to be 3.13% for the slope and 45.93% for the intercept, of the chromatographic method is 96.87% and 54.04% respectively. The sensitivity found for the method was 0.558 Eq. retinol/mL for the detection limit and 1.106 of Eq. retinol/mL for the quantification limit. One can see that the accuracy of the chromatographic system (1.86%) is below the minimum acceptable precision value of a HPLC chromatographic method (2.77%), indicating that the methodology used for the quantification of β-carotene meets the required analytical requirements of accuracy. Furthermore, it was estimated that the accuracy of the method was 96.07%, obtaining β-carotene recoveries of 103.93% with a confidence interval of 1.78%, indicating that the chromatographic methodology also meets the required analytical requirements of accuracy. Similarly, it was determined that the accuracy of the extraction method for β-carotene in the sample had a precision of 5.99% with a confidence interval of 5.71%, the accuracy of the extraction method for β-carotene in the sample was higher than the required minimum acceptable value (1.90%).
Guavas of the Palmira ICA I and regional blanca varieties have β-carotene concentrations close to the detection limit of the chromatographic method, while the regional roja variety fruits have higher β-carotene concentrations. During the ripening process of regional roja fruits, it was observed that β-carotene content increased until the mature stage and diminished during the senescence stage of the fruit, while in the regional blanca and Palmira ICA I varieties, b-carotene content increases throughout the entire process of fruit ripening (Fig.5). The highest concentration of β-carotene in the guava fruit is found in the regional roja variety, followed by the Palmira ICA I variety, and the lowest β-carotene concentration is found in the regional blanca variety. One can establish that fruits that had redder colors in the mesocarp (regional roja and Palmira ICA I varieties) had higher concentrations of carotenoids than in the regional blanca variety which was white in the mesocarp during the maturity process.
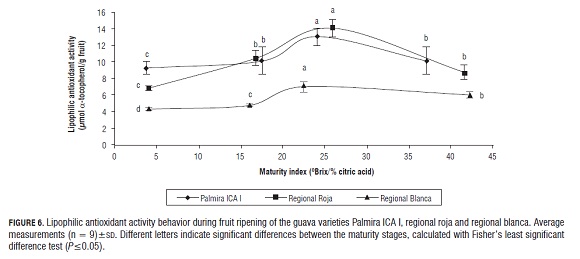
The results may also suggest that the red mesocarp in the regional roja variety is mainly due to the presence of carotenoids, including β-carotene, while the red color in the Palmira ICA I variety may be associated with other carotenoid compounds, with lycopene having the highest concentration (Wilberg and Rodríguez, 1995). Lipophilic antioxidant activity as measured with the β-carotene bleaching method showed that the regional roja and Palmira ICA I varieties had the highest lipophilic antioxidant activity, while the lowest antioxidant activity was observed in the regional blanca variety (Fig.6). It was also observed that as the maturity increases, lipophilic antioxidant activity increases until the mature stage, and decreases during the senescence stage in all guava varieties. Due to the similarity between the behaviors of lipophilic antioxidant activity and β-carotene concentration during the entire maturation process, linear correlations were established to determine the contribution of β-carotene to antioxidant activity of different varieties of guava fruits. We found that the correlation coefficient was higher in the regional roja variety (r2 = 0.830), while in the Palmira ICA I (r2 = 0.112) and regional blanca (r2 = 0.002) varieties a significant linear correlation was not found. This result suggests that β-carotene helps the lipophilic antioxidant activity of the regional roja guava fruit variety, while in the guava fruit varieties Palmira ICA I and regional blanca, antioxidant activity may be influenced by other carotenoid compounds that were observed at higher concentrations in the chromatographic profiles of the samples (Wilberg and Rodríguez, 1995).Conclusions
With this study, it was determined that the increased use of nutritional and functional properties such as lipophilic antioxidant activity of guava fruit is in the mature stage (about 25.96°Brix/% citric acid), where one sees the highest β-carotene concentration and lipophilic antioxidant activity, and that the regional roja and Palmira ICA varieties have lipophilic antioxidant activity that is very high compared to the regional blanca variety; and furthermore, that the lipophilic antioxidant activity of the regional roja variety is strongly influenced by β-carotene content, whereas in the Palmira ICA I and regional blanca varieties, lipophilic antioxidant activity may be more influenced by other carotenoid compounds that were observed in higher concentrations in the chromatographic profiles.
Acknowledgements
The authors are thankful for the financial support of the Ministerio de Agricultura y Desarrollo Rural de Colombia (Ministry of Agriculture and Rural Development) and Asohofrucol through the project "Obtaining antioxidants and dietary fiber from the guava" from the research program "Development of promising functional products from the guava to strengthen the supply chain", and to the Dirección de Investigación, Bogota (DIB) of the Universidad Nacional de Colombia.
Literature cited
Ali, Z.M., L.H. Chin, and H. Lazan. 2004. A comparative study on wall degrading enzymes, pectin modifications and softening during ripening of selected tropical fruits. Plant Sci. 167, 317-327. [ Links ]
Alquezar, B., M.J. Rodrigo, and L. Zacarías. 2008. Regulation of carotenoid biosynthesis during fruit maturation in the red fleshed orange mutant Cara Cara. Phytochemistry 69, 1997-2007. [ Links ]
Bashir, H.A., A. Bakr, and A. Goukh. 2003. Compositional changes during guava fruit ripening. Food Chem. 80, 557-563. [ Links ]
Bassetto, E., A.P. Jacomino, A.L. Pinheiro. and R.A. Kluge. 2005. Delay of ripening of Pedro Sato guava with 1-methylciclopropene. Postharv. Biol. Technol. 35, 303-308. [ Links ]
Bueno, M. 1997. Collaborative study: determination of retinol and carotene by high performance liquid chromatography. Food Chem. 59, 165-170. [ Links ]
Burri, B.J. 1997. Beta-carotene and human health: a review of current research. Nutr. Res. 17, 547-580. [ Links ]
Carreño, J., A. Martínez, L. Almela, and J.A. Fernández-López. 1995. Proposal of an index for the objective evaluation of the colour of red table grapes. Food Res. Intl. 28(4), 373-377. [ Links ]
Duan, X., G. Cheng, E. Yang, C. Yi, N. Ruenroengklin, W. Lu, Y. Luo, and Y. Jiang. 2008. Modification of pectin polysaccharides during ripening of postharvest of banana fruit. Food Chem. 111, 144-149. [ Links ]
El-Buluk, R.E., E.E. Babiker, and A.H. El-Tinay. 1995. Biochemical and physical changes in fruits of four guava cultivars during growth and development. Food Chem. 54, 279-282. [ Links ]
El-Bulk, R.E., E.F. Babiker, and A.H. El-Tinay. 1997. Changes in chemical composition of guava fruits during development and ripening. Food Chem. 59, 395-399. [ Links ]
Fonseca, S.C., F.A. Oliveira, and J.K. Brecha. 2002. Modelling respiration rate of fresh fruits and vegetables for modified atmosphere packages: a review. J. Food Eng. 52, 99-119. [ Links ]
Gartner, D.M. and P. Restrepo. 1997. Determinación de vitamina A por cromatografía líquida de alta eficiencia (CLAE) en bienestarina cruda. Rev. Colomb. Quím. 26, 31-41. [ Links ]
Mercado-Silva, E., P. Benito-Bautista, and M.A. García-Velasco. 1998. Fruit development, harvest index and ripening changes of guavas produced in central Mexico. Postharv. Biol. Technol. 13, 143-150. [ Links ]
Miller, N.J., J. Sampson, L.P. Candeias, P.M. Bramley, and C. Rice- Evans. 1996. Antioxidant activities of carotenes and xanthophylls. FEBS Lett. 384, 240-242. [ Links ]
Montalvo, E., H.S. García, B. Tovar, and M. Mata. 2007. Application of exogenous ethylene on postharvest ripening of refrigerated Atulfo mangoes. Food Sci. Technol. 40, 1466-1472. [ Links ]
Muruyama, H., T. Takahashi, R. Honda, and T. Fukushima. 1998. Cell wall changes in pear fruit softening on and off the tree. Postharv. Biol. Tecnol. 14, 143-149. [ Links ]
Nerd, A. and Y. Mizrahi. 1999. The effect of ripening stage on fruit quality after storage of yellow pitaya. Postharv. Biol. Technol. 15, 99-105. [ Links ]
Palomar, X., I. Roig, D. Grima, and M. Vendrell. 2005. Effects of nitrous oxide (N2O) treatment on the postharvest ripening of banana fruit. Postharv. Biol. Technol. 36, 167-175. [ Links ]
Pérez-Gutiérrez, R.M., S. Mitchell, and R. Vargas-Solis. 2008. Psidium guajava: A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 117, 1-27. [ Links ]
Quevedo, R., O. Díaz, B. Ronceros, F. Pedreschi, and J.M. Aguilera. 2009. Description of the kinetic enzymatic browning in banana (Musa cavendish) slices using non uniform color information from digital images. Food Res. Intl. 42(9), 1309-1314. [ Links ]
Ravindra, M.R. and T.K. Goswami. 2008. Modelling the respiration rate of green mature mango under aerobic conditions. Biosyst. Eng. 99, 239-248. [ Links ]
Saradhuldhat, P. and R.E. Paull. 2007. Pineapple organic acid metabolism and accumulation during fruit development. Scientia Hort. 112, 297-303. [ Links ]
Singh, S.P. and R.K. Pal. 2008. Response of climacteric-type guava (Psidium guajava L.) to postharvest treatment with 1-MCP. Postharv. Biol. Technol. 47, 307-314. [ Links ]
Velioglu, Y.S., G. Mazza, L. Gao, and B.D. Oomah. 1998. Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J. Agric. Food Chem. 46, 4113-4117. [ Links ]
Villanueva, M.J., M.D. Tenorio, M.A. Esteban, and M.C. Mendoza. 2004. Compositional changes during ripening of two cultivars of muskmelon fruits. Food Chem. 87, 179-185. [ Links ]
Wilberg V.C. and D.B. Rodríguez. 1995. HPLC quantitation of major carotenoids of fresh and processed guava, mango and papaya. Lebensm. Wiss. Technol. 28, 474-480. [ Links ]
Wilson, C.W., P.E. Shaw, and C.W. Campbell. 1982. Determination of organic acids and sugars in guava (Psidium guajava L.) by high performance liquid chromatography. J. Sci. Food Agric. 33(8), 777-780. [ Links ]
Xu, F., Q.P. Yuan, and H.R. Dong. 2006. Determination of lycopene by high performance liquid chromatography using sudan I as internal standard. J. Chromatogr. B 838, 44-49. [ Links ]