Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Agronomía Colombiana
Print version ISSN 0120-9965
Agron. colomb. vol.30 no.3 Bogotá Sept./Dec. 2012
PROPAGATION & TISSUE CULTURE
Efficient micropropagation of french tarragon (Artemisia dracunculus L.)
Micropropagación eficiente de estragón francés (Artemisia dracunculus L.)
John Cristhian Fernández-Lizarazo1 and Teresa Mosquera-Vásquez2
1Agricultural Engineering Programme, Faculty of Agricultural Sciences, Universidad de La Salle. Yopal (Colombia).jofernandez@unisalle.edu.co.
2Department of Agronomy, Faculty of Agronomy, Universidad Nacional de Colombia. Bogota (Colombia).
Received for publication: 30 July, 2011. Accepted for publication: 30 October, 2012.
ABSTRACT
French tarragon is cultivated in Colombia for exportation. According to French tarragon producers, crops should be renovated but traditional propagation with cuttings has a high percentage of loss and there is incidence of Colombian tarragon crop rust. An alternative for inducing juvenile characteristics and increasing percentages of rooting and clean plant propagation material is micropropagation. The aim of this research was to generate an efficient micropropagation protocol, which would be useful for providing clean propagation plant material and contributing to the renovation of French tarragon crops in Colombia. For establishment, the effect of both type and concentration of gelling agents and liquid mediums was assessed. For multiplication and rooting, the effect of indole acetic acid (IAA), N6-furfuril amine purine, planting orientation and pinching was assessed. Although French tarragon showed susceptibility to hyperhydricity, it was possible to determine protocols from 1 mm-meristematic tips established in the liquid medium Murashige and Skoog (MS), with in vitro multiplication/ rooted in a solid medium MS supplemented with IAA 0.1 mg L-1. Additionally, an alternative multiplication protocol was assessed from propagules horizontally sown in a solid medium MS supplemented with KIN 0.5 mg L-1 and AIA 5 mg L-1.
Key words: micropropagation, indole acetic acid, N6-furfuril amine purine, hyperhydricity, pinching.
RESUMEN
El estragón francés es cultivado en Colombia con fines de exportación. Según observaciones de los productores de estragón francés, los cultivos deben renovarse pero la propagación tradicional por esquejes tiene un alto porcentaje de pérdida y hay incidencia de la roya en los cultivos colombianos. Una vía alterna para inducir características de juvenilidad, incrementar porcentajes de enraizamiento y limpiar el material vegetal de propagación es la micropropagación. Esta investigación tuvo como propósito generar un protocolo de micropropagación eficiente útil para proveer material vegetal de propagación que contribuya a la renovación de los cultivos de estragón francés en Colombia. Para el establecimiento, se evaluó el efecto del tipo y concentración de agentes gelificantes y del medio líquido. Para la multiplicación, se evaluó el efecto del ácido indol acético (AIA), de la N6-furfuril amino purina, de la orientación del propágulo y del despunte. Aunque el estragón mostró susceptibilidad a la hiperhidratación, fue posible determinar protocolos a partir de puntas meristemáticas de 1 mm establecidas en medio líquido Murashige and Skoog (MS) y multiplicadas/ enraizadas in vitro en medio sólido MS suplementado con AIA 0,1 mg L-1. Adicionalmente se determinó un protocolo alterno de multiplicación a partir de propágulos sembrados horizontalmente en medio sólido MS y suplementado con KIN 0,5 mg L-1 y AIA 5 mg L-1.
Palabras clave: micropropagación, ácido indol acético, N6- furfuril amino purina, hiperhidratación, despunte.
Introduction
French tarragon comes from eastern Russia, but it is distributed in crops primarily in Central Europe and South and North America (Small and Deutsch, 2001). In Colombia, French tarragon is one of the most important culinary plants for exportation and there are crops over 8-yearsold, although Bareño and Clavijo (2006), recommended renovation of plants every 3 or 4 years.
One of the purposes of micropropagation is to improve morphological and biochemical qualities of certain plants, especially medicinal, aromatic and timber crops; in the case of Salvia officinalis (Avato et al., 2005; Santos et al., 2002), Salvia fruticosa (Arikat et al., 2004), Artemisia annua (Liu et al., 2003), Acacia mearnsii (Beck et al., 1998), Vitis vinifera (Heloir et al., 1998), Cynara scolymus L. (Tabazza et al., 1998), Corylus avellana (Rey et al., 1998), it has been found that juvenile qualities obtained from micropropagation express, in a species-specific way, better rooting capacity, change in leaf morphology, change in amount of trichomes, variation in polyamide content, antioxidant activity, increased capacity of essential oils and greater vigor, etc. In the case of mature crops of French tarragon, juvenile induction, which is only possible under in vitro conditions (Pedroza, 2008), is important because its sexual reproduction characteristics are quite limited (Mackay and Kitto, 1988) especially in tropical areas (Bareño, 2006).
On the other hand, French tarragon crops in Colombia have two principal difficulties: absences of alternative propagation techniques and limiting diseases. Propagation of French tarragon is traditionally performed with cuttings, which has 50% loss due to rooting difficulty. This difficulty increases the cost of the procedure and decreases the quantity of plants for the market (Bareño, 2006). Additionally, the same author reports that in Colombia, the most limiting disease of this crop is rust caused by the fungus Puccinia tanaceti D.C. var. dracunculina with losses that can reach 70% in a period of 15 d.
Micropropagation is an alternative tool of propagation that makes it possible to avoid systemic pathogens (Bhojwani and Razdan, 1996). One of the most important techniques to obtain pathogen free plants is the culture of meristematic tips (Pedroza, 2008). MacKay and Kitto (1988) reported a nine-week protocol for the in vitro propagation of tarragon cuttings that were 15 mm in length, however, the size of the explants did not guarantee clean plant material. In this sense, the aim of this research was to generate an efficient micropropagation protocol that could provide clean propagation plant material to contribute to the renovation of French tarragon crops in Colombia.
Materials and methods
Plant material
French tarragon cuttings were provided by the Agronomy Faculty of the National University of Colombia. 20 mm length cuttings were selected with no apparent presence of rust pustules (P. tanaceti D.C. var. dracunculina [Fahrend.]) Cummis, however, this disease was present in the crop of origin (Gamba and Ramírez, 2005).
Methodology
This research developed multiple trials aimed at obtaining efficient protocols for the establishment, multiplication and rooting of French tarragon.
Establishment
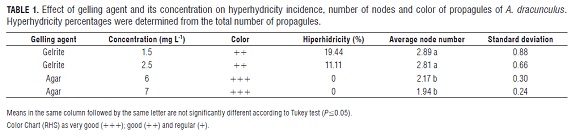
The cuttings were disinfected employing the Mackay and Kitto(1988) protocol. Leaflets were removed to obtain 1mm long tips. The medium used was Murashige and Skoog (MS), without plant growth regulators (PGR's) and gellified with Difto Bacto Agar, Oxoid® 0.6%. Since hyperhydricity was present in preliminary trials, the effect of the agar (0.6 and 0.7%) and gelrite (0.15 and 0.25%) on the percentage of hyperhydricity was assessed. Three meristematic tips constituted an experimental unit and 12 experimental units per treatment were planted.
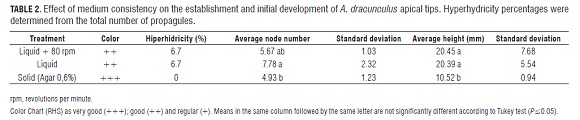
Finally, this experiment compared the effect of a liquid medium on initial development of French tarragon explants using three treatments: liquid MS without agitation (5 mL); liquid MS with orbital agitation at 80 rpm (5 mL); and solid MS gellified with Difto Bacto Agar, Oxoid® 0.6% as the control treatment. Five meristematic tips constituted an experimental unit and five experimental units per treatment were planted 50 mL glass bottles were used.
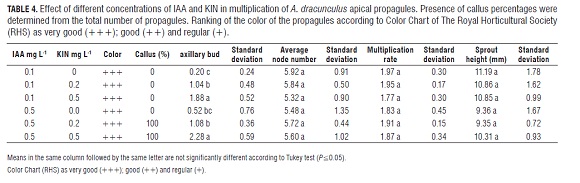
The cultivation grew in a growth chamber with a photoperiod of 16 h of light and a constant temperature of 25°C. Evaluations were done the third week after sowing. Average node number, height, and color of propagules were assessed employing the Color Chart of The Royal Horticultural Society (RHS), structuring three codes for the green color quality of propagules. Those codes were: 1) green group 14 1b as very good (+++); 2) green group 142b as good (++) and 3) green group 150 c as regular (+).
Multiplication
The Mackay and Kito (1988) protocol suggested, for French tarragon micropropagation, the use of benzyl adenine (BA) and naftalenacetic acid (NAA), which are repeatedly associated with hyperhydricity (Fernández, 2009). Some authors report that NAA and particularly BA, promote abnormal development (Debergh, 1983; Kataeva et al., 1991; Vandemortele, 2001). Vankova et al. (1991) claimed that it is possible to control hyperhydricity by modulating types of internal cytokinins trough the nature of applied synthetic cytokinins. In this sense, this study evaluated the effect of other growth regulators less associated with hyperhydricity, such as IAA and KIN.
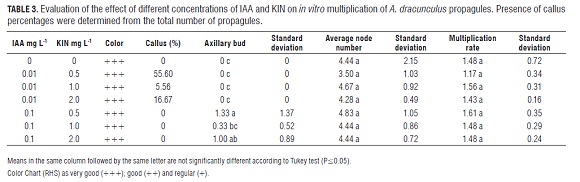
Initially, seven treatment combinations were evaluated: MS media culture supplemented with the growth regulators indole acetic acid (IAA) (0.01 and 0.1 mg L-1) and N6- furfuril amine purine (Kinetin, KIN) (0.5; 0.1 and 2.0 mg L-1); in the control treatment growth regulators were not used (Tab.3). Three node apical cuttings were vertically planted per experimental unit with six experimental units per treatment.
Based on the results of the previous test, a second assay was carried out which evaluated two types of propagules. First, a cutting of three apical buds was planted vertically and second, a cutting of one axiliary node was planted horizontally.
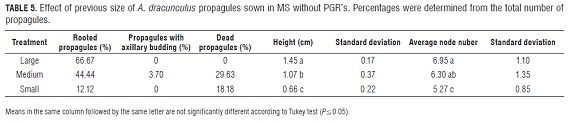
For each of the two types of propagules, six treatment combinations were evaluated. MS media, supplemented with the growth regulators IAA (0.1 and 0.5 mg L-1) and KIN (0, 0.5 and 0.2 mg L-1), were used. As the control, IAA 0.1 mg L-1 plus KIN 0.5 mg L-1 was used because of the results of the previous test. Five propagules were planted per experimental unit with five experimental units per treatment. In the case of axillary buds, they were planted horizontally. Due to the high variability of individual plant responses within different previous experiments, a test of individual plants was conducted. In order to reduce the effect of exogenous growth regulators of previous treatments, the propagules were planted in MS without PGRs for two weeks. Three types of propagules were grouped and used: large (≥ 25 mm), medium (24 mm ≥ x ≥ 11 mm) and small (≤ 10 mm). These propagules were cut to a size of 5 mm and sown in MS without PGRs to determine if they retained their characteristics of growth, forming three treatments. Each experimental unit consisted of three propagules and used seven, nine and eleven replications for large, medium and small treatments, respectively. The evaluation was conducted in the second week after sowing.
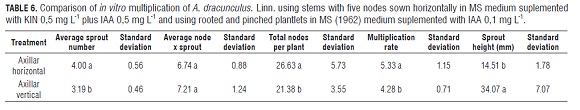
Once a homogenous group of in vitro rooted plants was obtained, an assay to optimize multiplication was carried out. 1) stems with five nodes laid horizontally in MS, supplemented with KIN 0.5 mg L-1 and IAA 0.5 mg L-1 (based on results of this research) and, 2) three weeks in vitro with rooted and newly pinched plantlets, leaving five nodes per stem, in MS without growth regulators. Four propagules constituted an experimental unit, with eight experimental units evaluated per treatment.
In all the trials, a MS medium supplemented with 3% sucrose and Difto Bacto Agar, Oxoid®, 0.6%, and a pH of 5.7 ± 0.05 was used. The following variables were measured, as appropriate: height of propagule, number of nodes, rooted propagules, propagules with an axillary shoot and dead propagules, color of propagules, percentage of propagules with calli, proliferation of propagules and multiplication rate with the following formula:
Multiplication rate = number of final nodes (3 weeks)/ number of initial nodes (1)
Rooting tests were not done because under the selected multiplication treatments, an additional in vitro rooting was obtained (see results). ANOVA was used with SAS version 9.1 under a completely random model (P≤0.05) and means were compared using Tukey´s test.
Acclimatization
Acclimatization was carried out in a greenhouse, using plantlets rooted in vitro with 12-14 leaves. Transplantation was performed in 96 alveoli trays and as the substrate, a sterile peat was used. The trays were kept in propagation banks with 70% penetration of light. One minute frequency nebulizer irrigation was applied to maintain 80% relative humidity. This frequency gradually declined over two weeks until the acclimatization was completed.
Results
Establishment
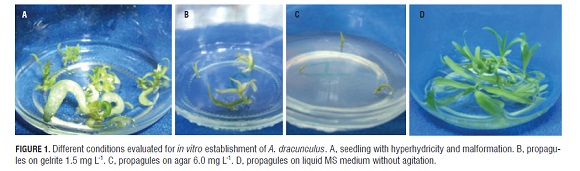
The propagules sown in agar did not have hyperhydricity (Fig.1 a), while gelrite increased propagules with hyperhydricity at rates between 11.1 and 19.4% (Tab.1, Fig.1 b-1 c). On the other hand, gelrite apparently enabled the development of more nodes and at a greater size than agar (Tab.1). However, gelrite induced malformations and decreased the intensity of the green color in comparison with the agar. This was possibly due to loss of photosynthetic pigment (Fig.1 b).
Meristematic tips planted in a liquid medium developed more nodes, were taller, but had less intense photosynthetic pigment and a 6.7% percentage of hyperhydricity (Tab.2, Fig.1 d), this percentage was lower than the percentage of hyperhydricity when using gelrite (Tab.1).
Multiplication
In the first multiplication assay, color, multiplication rate, and average of nodes developed by propagules did not have statistically significant differences. However, higher values obtained in these parameters corresponded to the treatment with IAA 0.1 mg L-1 plus KIN 0.5 mg L-1 (Tab.3).
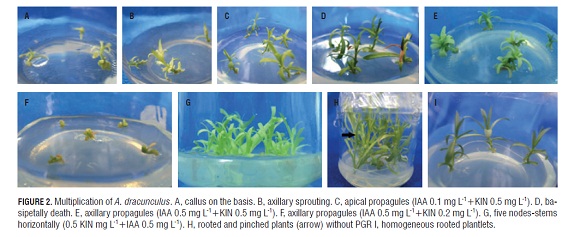
Differences between the treatments IAA 0.01 mg L-1 and IAA 0.1 mg L-1 were observed. In the presence of IAA 0.01 mg L-1, a percentage ranging from 5.56 to 55.6% of the propagules developed calli at the bottom (Fig.2 a) and none of them developed proliferation of axillary buds. On the contrary, in the presence of IAA 0.1 mg L-1, propagules developed a proliferation of axillary buds but none of them developed calli at the base (Tab.3, Fig.2 b). The treatment with IAA 0.1 mg L-1 plus KIN 0.5 mg L-1 was chosen as the control of the next assay (Tab.4), because while it presented a greater proliferation of axillary buds, it had the highest multiplication rate (Tab.3).
In the case of apical bud propagules planted vertically, there were no statistically significant differences in most variables (Tab.4), however, it was noted that IAA 0.1 mg L-1 prompted a higher number of nodes and taller propagules than all the other treatments (Fig.2 c), moreover, in this treatment, 100% rooting was observed on the 13th d after sowing. In addition, IAA 0.5 mg L-1 plus KIN 0.2 or 0.5 mg L-1 induced calli at the base of the plants (Tab.4). On the other hand, a basipetal death of the seedlings was observed (Fig.2 d). This phenomenon presented in treatments with KIN plus IAA but not with IAA alone.
In the axillary bud propagules planted horizontally, no statistically significant differences were presented, but some qualitative differences became apparent. The medium supplemented with IAA 0.5 mg L-1 plus KIN 0.5 mg L-1 induced more leaf development and vigor (Fig.2 e) compared to the other treatments, in spite of that, the propagules developed a similar number of nodes (Fig.2 f).
An analysis of individual assessment showed taller and rooted plants for the larger propagule treatment, while there was a greater number of deaths in the medium and small propagules. In addition, a tendency to proliferate axillary buds was observed, accompanied by hyperhydricity and death in the medium propagules and, more so, in the small propagules (Tab.5).
Axillary propagules planted horizontally in a medium supplemented with KIN 0.5 mg L-1 plus IAA 0.5 mg L-1 induced fewer nodes per shoot but more shoots per explant compared to plants rooted and cultured without PGRs and recently pinched (Tab.6). The number of shoots per plant was the variable which determined the highest number of total nodes per plant and therefore, the highest rate of multiplication in horizontally sown explants. However, these buds presented 2.4 times less than average size than shoots from rooted plants (Tab.6 and Fig.2 g-h).
All seedlings cultured in a medium supplemented with IAA 0.1 mg L-1 were developed in a homogeneous way (Fig.2i) with roots present (100%) at the 13th d after sowing. The acclimatization of the seedlings with the methodology referred to this research allowed for 95% survival of plant material.
Discussion
Establishment
Hyperhydrated French tarragon propagules had a glassy aspect, noticeable malformations and an apparent decrease in photosynthetic pigments (Fig.1 a), as has also been reported by Mackay and Kitto (1988). These kinds of changes have been thoroughly characterized (Kevers et al., 1984; Franck et al., 1998a; Franck et al., 1998b; Gribble et al., 1998; Franck et al., 2001; Park et al., 2004). In this experiment, the factors which induced hyperhydricity in A. dracunculus propagules could have come from the explants or hormonal imbalances, because the plant material used in this research came from a crop with an incidence of tarragon rust (P. tanaceti D.C. var. dracunculina) (Fahrend.) (Cummis) (Gamba and Ramírez, 2005) and the stress induced by pathogens can manifest in abnormal growth due to hormonal imbalances (Pessarakli,1999).
Changes in hyperhydricity of A. dracunculus were not evident when the concentration of each gelling agent increased (Tab.6). In contrast, the literature reports that the traditional gelling agent concentration has been increased in order to decrease propagule hyperhydricity (Brand, 1993; Deberg et al., 1981; Debergh 1983; Williams and Taji, 1991). Additionally, only the propagules cultured in the Difto Bacto Agar did not present hyperhydricity, but they had fewer nodes than those cultured in gelrite (Tab.1).
Hyperhydricity can decrease according to the type of gelling agent (Tab.1), from gelrite to Difto Bacto Agar, which provides a harder medium and less matrix potential, making materials that potentially may induce hyperhydricity less available. The physical structure of the gelrite seems to allow increased absorption of substances which may be responsible for hyperhydricity, such as cytokinins, ammonium ions and water (Williams and Taji, 1991). Moreover, The brand used in this research (Oxoid®) produced efficient results in hyperhydricity management as well as in multiplication. This product influences water retention capacity of propagules since it promotes a better "pumping function". It seems to allow better absorption of water and nutrients (Beruto et al., 1999).
Liquid medium increased the number of nodes, average height and reactivated growth of tarragon propagules (Tab.2, Fig.1 d); and it possibly facilitated rapid absorption of nutrients, faster initial development of propagules and especially of the vascular system, which would therefore, facilitate the possible hyperhydricity evasion at initial growth under in vitro conditions. Similar to A. dracunculus, Artemisia judiaca was massively propagated using solid, liquid and liquid with supplements media; reportedly the liquid medium had the best results for the spread of buds (Liu et al., 2004).
Employment of liquid media with agitation produced a smaller average node number and no significant variations on average height of propagules in comparison with liquid medium without agitation, suggesting that adding 5 mL of liquid medium in containers used in this test does not affect oxygen availability for propagules, allowing their normal development.
Multiplication
Results suggest that the presence of calli is dependent on type, interaction, and concentration of PGRs because, they occur with KIN, in assessed concentrations, with the addition of IAA 0.01 mg L-1 and 0.5 mg L-1, but not with the addition of IAA 0.1 mg L-1 (Tab.3 and 4). Furthermore, auxin enhanced the effect of cytokinin, inducing proliferation of axillary buds, i.e. the treatments with IAA alone had the lowest percentages of proliferation of axillary buds, which increased with increased concentrations of KIN and IAA (Tab.4).
Additionally, in the second multiplication assay of apical propagules, basipetal death was observed in treatments supplemented with IAA 0.1 mg L-1 plus KIN 0.2 mg L-1; IAA 0.1 mg L-1 plus KIN 0.5 mg L-1; IAA 0.5 mg L-1 plus KIN 0.2 mg L-1; and IAA 0.5 mg L-1 plus KIN 0.5 mg L-1 (Tab.4); possibly due to the exogenous supplementation of KIN, which possibly reduced apical dominance and caused a subsequent apical necrosis (Fig.2 d).
On the other hand, both the vigor and multiplication rate of propagules from an axillary origin had a tendency to be greater when cultured in media with increased concentrations of IAA 0.5 mg L-1 and KIN 0.5 mg L-1 (Fig.2 e-f), which might result from a different hormone balance compared with apical propagules.
Response variations of in vitro plants of different sizes may be caused by previous hormonal differences which, as in the apple, may be due to a temporary physiological state (Puente and March, 1997). For example, propagules of a medium-sized origin (24 mm ≥ x ≥ 11 mm) were more heterogeneous than those of a large or small size, including others intermediate features (Tab.5). This could be due to the fact that, during the selection process in field, cuttings that were designated for micropropagation were at an intermediate physiological range that was not easily distinguishable even when they were collected at the same size (Fig.1 c).
The multiplication assay using uniform plants, five node explants planted horizontally in a medium supplemented with KIN 0.5 mg L-1 plus IAA 0.5 mg L-1 showed a more efficient multiplication rate in comparison with plantlets rooted and pinched (Tab.6).
The average multiplication rate (5.33) using five node explants horizontally planted (Tab.6) was 1.5 times greater than that observed in three node explants at the same concentration of PGRs (3.7) (data not shown) and 1.3 times higher than the plantlets rooted and pinched in this test (Tab.6). This increase may be due to the fact that the surface contact with the nutrient medium increased when the explant was larger, allowing absorption of larger quantities of water, nutrients and PGRs. This effect had already been reported in French tarragon by Mackay and Kitto (1988).
In addition, due to the position of the explant, the probability of each node getting assimilates is similar and therefore competition between sinks decreases (Fig.2 g), which is reflected in the highest average number of nodes per explant (Tab.6). Stems used in this test came from more developed plants than in previous trials with horizontal explants, which involved further development of the vascular system, greater differentiation of tissues and therefore, greater capacity and efficiency in nutrient uptake.
The observed elongation in sprouts from plantlets rooted and pinched in vitro (Fig.2 h) is explained by lower apical dominance due to pinch and because growth was supported by water and nutrients uptake, specifically by roots. The root, as a structure for this function, positively affects efficiency in both biomass formation and organogenesis. Another explanation may be that rooted seedlings had better initial proliferation due to the effect of the cytokinins sent from the root (Puente and March, 1997). In addition, competition for nutrients did not allow all the buds per stem to develop, but those that developed, elongated because they had increased availability of nutrients per node and the density of stems (sprouts) generated competition for light.
The results suggest that the use of KIN 0.5 mg L-1 and IAA 0.5 mg L-1 can be more efficient in the multiplication rate (Tab.6), however, the lack of sprout elongation would require a greater number of transplants in order to get suitable in vitro rooted plants, as well as, more laboratory inputs and PGRs. Conversely, plantlets that are rooted in vitro and previously pinched have a lower rate of multiplication but larger length between nodes. These facts make it possible to obtain suitable plants, with adequate size, for subsequent multiplication or rooting without additional transplants and with fewer laboratory inputs and without additional PGRs.
MacKay and Kitto (1988) reported a methodology based on the establishment of 15 mm long French tarragon cuttings using a multiplication protocol that induced strong axillary sprouting using a MS and BAP 0.4 mg L-1 plus NAA 0.05 mg L-1. Those authors, however, did not report on in vitro but ex vitro rooting with the addition of NAA 1 mg L-1. These authors claimed that the time of in vitro establishment/ proliferation is four weeks and for field rooting, five weeks, for a possible total required time of nine weeks.
In contrast, this research used 1 mm meristematic tips. Initially, the apical dominance was stimulated. This stimulus was obtained with the initial use of a liquid MS (1962) medium and then a solid MS medium, supplemented with only IAA 0.1 mg L-1, i.e. with fewer gelling agents and growth regulators.
Finally, the in vitro establishment time was three weeks. The in vitro multiplication/rooting was three weeks and according to the standard hardening protocol, requiring two to three weeks. The total time required from in vitro establishment to hardened rooted plants in the field is about eight to nine weeks.
Conclusion
This research generated a protocol to micropropagate French tarragon (A. dracunculus L.) that consists of an in vitro establishment of 1 mm-meristematic tips in a liquid MS medium and in vitro multiplication/rooting in a solid MS medium supplemented with IAA 0.1 mg L-1. An alternative multiplication protocol was assessed for propagules horizontally sown in a solid MS medium supplemented with KIN 0.5 mg L-1 and AIA O.5 mg L-1.
This protocol contributes to the renovation of Colombian French tarragon crops through the production of more vigorous plant propagation material with less inoculum pressure from pathogens, and with a similar time period as that of the currently reported protocols.
Literature cited
Avato, P., I. Morone, and C. Rutaand D'Elia. 2005. Glandular hairs and essential oils in micropropagated plants of Salvia officinalis L. Plant Sci. 169(1), 29-36. [ Links ]
Arikat, N., F. Jawad, N. Karam, and R. Shibli. 2004. Micropropagation and accumulation of essential oils in wild sage (Salvia fruticosa Mill.). Scientia Hort. 100, 193-202. [ Links ]
Bareño, P. 2006. Estragón (Artemisia dracunculus). pp. 97-99. In: Clavijo, J., Bareño, P., Chaparro, L. and C. Guido (eds.). Últimas tendencias en hierbas aromáticas culinarias para exportación en fresco. Universidad Nacional de Colombia, Bogota. [ Links ]
Bareño, P. and J. Clavijo. 2006 Hierbas aromáticas culinarias para exportación en fresco. pp. 7-9. In: Clavijo, J., P. Bareño, L. Chaparro, and C. Guido. (eds.). Últimas tendencias en hierbas aromáticas culinarias para exportación en fresco. Universidad Nacional de Colombia, Bogota. [ Links ]
Beck, S., R. Dunlop, and J. Van Staden. 1998. Rejuvenation and micropropagation of adult Acacia mearnsii using coppice material. Plant Growth Reg. 26(3), 149-153. [ Links ]
Beruto, M., P. Curirand, and P. Debergh. 1999. Influence of agar on in vitrocultures: II. Biological performance of Ranunculus on media solidified with three different agar brands. In vitro Cell. Dev. Biol.-Plant 35, 94-101. [ Links ]
Bhojwani, S. and M. Razdan. 1996. Plant tissue culture: theory and practice, a revised edition. Elsevier, Amsterdam. [ Links ]
Brand, M. 1993. Agar and ammonium nitrate influence hyperhydricity, tissue nitrate and total nitrogen content of serviceberry (Amelanchier arborea) shoots. In vitro Plant Cell Tissue and Org. Cult. 35(3), 203-209. [ Links ]
Debergh, P., Y. Harbaouiand, and R. Lemeur. 1981. Mass propagation of globe artichoke (Cynara scolymus): evaluation of different hypotheses to overcome vitrification with special reference to water potential. Physiol. Plant. 53(2), 181-187. [ Links ]
Debergh, P. 1983. Effects of agar brand and concentration on the tissue culture medium. Physiol. Plant. 59(2), 270-276. [ Links ]
Fernández, J. 2009. Evaluación del efecto de la micropropagación en el enraizamiento y producción de biomasa de estragón (Artemisia dracunculus Linn.) en campo a través de un modelo de crecimiento. M.Sc. thesis. Faculty of Agronomy, Universidad Nacional de Colombia, Bogota. [ Links ]
Franck, T., M. Crèvecoeur, J. Wuest, H. Greppinand, and T. Gaspar. 1998a. Cytological comparison of leaves and stems of Prunusavium L. shoots cultured on a solid medium with agar or gelrite. Biotech. Histochem. 73(1), 32-43. [ Links ]
Franck, T., C. Kevers, C. Penel, H. Greppin, J. Hausmanand, and T. Gaspar. 1998b. Reducing properties, and markers of lipid peroxidation in normal and hyperhydrating shoots of Prunusavium L. J. Plant Physiol. 153, 339-346. [ Links ]
Franck, T., T. Gaspar, C. Kevers, C. Penel, J. Dommesand, and J. Hausman. 2001. Are hyperhydric shoots of Prunusavium L. energy deficient?. Plant Sci. 160, 1145-1151. [ Links ]
Gamba, Y. and N. Ramírez. 2005. Aspectos biológicos de la roya del estragón Puccinia tanaceti D.C. var. dracunculina (Fahrend) Cummins. M.Sc. thesis. Faculty of Agronomy, National University of Colombia, Bogota. [ Links ]
Gribble, K., J. Tingle, V. Sarafis, A. Heaton, and P. Holford. 1998. Position of water in vitrified plants visualised by NMR imaging. Protoplasma 201, 110-114. [ Links ]
Heloir, M., J. Fournioux, M. Barbier, P. Jeandet, and R. Bessis. 1998. Endogenous polyamine concentrations in juvenile, adult and micropropagated grapevine (Vitis vinifera L.) cv. Pinot noir). Vitis 37, 61-62. [ Links ]
Kataeva, N., I. Alexandrova, R. Butenko, and E. Dragavtceva. 1991. Effect of applied and internal hormones on vitrification and apical necrosis of different plants cultured in vitro. Plant Cell Tiss. Org. Cult. 27(2), 149-154. [ Links ]
Kevers, C., M. Coumans, MF. Coumans-Gillèsand, and T. Caspar. 1984. Physiological and biochemical events leading to vitrification of plants cultured in vitro. Physiol. Plant. 61(1), 69-74. [ Links ]
Liu, C.Z., C. Guo, Y. Wang. and F. Ouyang. 2003. Comparison of various bioreactors on growth and artemisinin biosynthesis of Artemisia annua L. shoot cultures. Process Biochem. 39, 45-49. [ Links ]
Liu, C.Z., S.J. Murch, M. Demerdashand, and P.K. Saxena. 2004. Artemisia judaica L.: micropropagation and antioxidant activity. J. Biotechnol. 110, 63-71. [ Links ]
Mackay, W. and S. Kitto.1988. Factors affecting in vitro shoot proliferation of French tarragon. Hort. Science113, 282-287. [ Links ]
Murashige, T. and F. Skoog. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Phisiol. Plant. 15, 473-497. [ Links ]
Park, S., J. Jeon, H. Kimb, Y. Park, C. Aswath, and H. Joung. 2004. Effect of sealed and vented gaseous microenvironments on the hyperhydricity of potato shoots in vitro. Scientia Hort. 99, 199-205. [ Links ]
Pedroza, J. 2008. Aplicaciones del cultivo de tejidos en condiciones in vitro. Universidad Distrital Francisco José de Caldas, Bogota. [ Links ]
Pessarakli, M. 1999. Handbook of plant and crop stress. 2nd ed. Marcel Dekker Inc, New York, YN. [ Links ]
Puente, J. and J. Marh, 1997. In vitro rootability of clonal apple microcuttings, derived from rooted and unrooted shoots. Scientia Hort. 68, 227-230. [ Links ]
Rey, M., C. Díaz, and R. Rodríguez. 1998. Free polyamine content in leaves and buds of hazelnut (Corylusavellana L. cv. Negret) trees subjected to repeated severe pruning. Scientia Hort. 76, 115-121. [ Links ]
Rousi, A. 1968. Cytogenetic comparison between two kinds of cultivated tarragon (Artemisia dracunculus). Hereitas 10, 194-213. [ Links ]
Santos, P., R. Seabra, P. Andrade, and M. Fernández. 2002. Phenolic antioxidant compounds produced by in vitro shorts os sage (Salvia officinales). Plant Sci. 162, 981-987. [ Links ]
Small, E. and G. Deutsch. 2001. Herbes culinaires pour nos Jardins de Pays. Froid Research Press, Ottawa, USA. [ Links ]
Tabazza, R., V. Papacchioliand, and G. Ancora. 1998. An improved medium for in vitro propagation of globe artichoke (Cynara scolymus L.) Tree Physiol. 18, 251-257. [ Links ]
Vandemortele, J., C. Kevers, J. Billard, and T. Gaspar. 2001. Osmotic pretreatment promotes axillary shooting from cauliflower curd pieces by acting through internal cytokinin level modifications. J. Plant Physiol. 158, 221-225. [ Links ]
Vankova, R., K. Hsiao, C. Bornman, and A. Gaudinova. 1991. Effects of synthetic cytokinins on levels of endogenous cytokinins and respiration patterns of Beta vulgaris cells in suspension. J. Plant Growth Reg. 10(1), 197-199. [ Links ]
Williams, R. and A. Taji. 1991. Effect of temperature, gel concentration and cytokinins on vitrification of Oleariamicrodisca (J.M. Black) in vitro shoot. Plant Cell Tiss. Org. Cult. 26(1), 1-6. [ Links ]