Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Agronomía Colombiana
Print version ISSN 0120-9965
Agron. colomb. vol.30 no.3 Bogotá Sept./Dec. 2012
SOILS, FERTILIZATION & MANAGEMENT OF WATER
Hydrolysis of cellulose and oil palm empty fruit bunches by using consortia of fungi isolated from the soil of Colombian high andean forest
Hidrólisis de celulosa y tusa de palma de aceite usando consorcios de hongos aislados del suelo del bosque alto andino colombiano
Luz Aida Moya A.1 and Esperanza Torres R.2
1Faculty of biological sciences, Universidad Militar Nueva Granada; Centro de Genómica y Bioinformática-GeBiX. Bogotá (Colombia). etorresr@unal.edu.co2Department of Agronomy, Faculty of Agronomy, Universidad Nacional de Colombia; Centro de Genómica y Bioinformática-GeBiX. Bogotá (Colombia).
Received for publication: 24 May, 2102. Accepted for publication: 30 October, 2012.
ABSTRACT
Hydrolytic activity was evaluated in a mixture of supernatants produced by filamentous fungi grown individually on microcrystalline cellulose and empty fruit bunches. The strains that were used correspond to two types of isolates; the first was made from soil samples from a transect of a high andean forest of Colombia, in the Parque Natural Nacional de Los Nevados, where, based on previous studies, we selected the strains B7, B11, B11M and B19. The second isolate was obtained from a pool of oil palm empty fruit bunches (Eleaeis guinensis Jacq.) in different states of decomposition on the Unipalma plantation located in the eastern plains; strains TA1 and TA2. To perform the hydrolysis of cellulose and empty fruit bunches, the previously obtained supernatants from each of the selected strains were cultivated for 300 hours in cellulose and characterized individually by endoglucanase, exoglucanase, and β-glucosidase activity. Individual supernatants were mixed at a 1:1 ratio to form consortia; and hydrolytic activity was evaluated in the substrates at two hours. The glucose concentration was determined by the 3,5-dinitrosalicylic acid (DNS) method. The results show that hydrolysis of empty fruit bunch to glucose was favored by three pools of supernatants, with increases greater than 400% in comparison with the hydrolysis obtained by individual supernatants, demonstrating the potential to decompose palm empty fruit bunches; thereby contributing to the reduction of decay time of empty fruit bunches and the decrease of environmental and health problems.Key words: consortia, enzymatic hydrolysis, empty fruit bunches, cellulases, filamentous fungi, biomass.
RESUMEN
Se evaluó la actividad hidrolítica a partir de la mezcla de sobrenadantes producidas por hongos filamentosos cultivados individualmente sobre celulosa microcristalina y tusa. Las cepas utilizadas corresponden a dos tipos de aislamientos, el primero se realizó a partir de muestras de suelo de un transepto del bosque altoandino colombiano del Parque Natural Nacional de Los Nevados, en donde con base en estudios previos, se seleccionaron las cepas B7, B11, B11M y B19. El segundo aislamiento se obtuvo a partir de un pool de tusas de palma de aceite (Eleaeis guinensis Jacq.) en diferentes estados de descomposición obtenidos de la plantación UNIPALMA ubicada en los Llanos Orientales, cepas TA1 y TA2. Para realizar la hidrólisis de tusa y celulosa, previamente se obtuvieron los sobrenadantes de cada una de las cepas seleccionadas a las 300 horas de cultivo en celulosa y se caracterizaron individualmente por su actividad endoglucanasa, exoglucanasa y β-glucosidasa. Se mezclaron los sobrenadantes individuales en una proporción 1:1 para formar los consorcios y se evaluó la actividad hidrolítica sobre los sustratos a las dos horas. La concentración de la glucosa se determinó por el método ácido 3,5-dinitrosalicílico DNS. Los resultados muestran que la hidrólisis de tusa a glucosa fue favorecida por tres consorcios de sobrenadantes, con aumentos mayores al 400% en comparación con la hidrólisis obtenida por los sobrenadantes individuales, lo cual demuestra su potencial para degradar tusas de palma. De esta forma se pretende contribuir a la reducción en el tiempo de descomposición de las tusas y disminuir la fuente de problemas ambientales y sanitarios.
Palabras clave: consorcios, hidrólisis enzimática, tusa, celulasas, hongos filamentosos, biomasa.
Introduction
The biological conversion of cellulose, the insoluble, linear homopolymer constituting more than 40% of the ground biomass, occurs through hydrolysis, chemical or biological, by breaking β-1,4-glucosidic bonds. Chemical methods involve the use of strong acids or bases in large quantities, which when released cause an environmental hazard (Singh et al. , 2010). In contrast, biological methods provide an alternative with the use of microorganisms or enzymes produced by same, which hydrolyze cellulose to obtain simpler molecules such as glucose.
Biological hydrolysis is generated by enzymes, cellulases, produced by highly diverse microorganisms, including, the most reported due to their ability to produce extracellular enzymes, the aerobic filamentous fungi Trichoderma reesei and Aspergillus niger, which play an important role in the recycling of this polymer in the biosphere (Yang et al. , 2011) and are used industrially in the production of cellulases for different uses.
Cellulases are complex enzyme systems consisting of three classes of enzymes: endoglucanases, exoglucanases and β-glucosidases, which are complementary and act synergistically during the degradation process (Lynd et al., 2002). Endoglucanase or CMCase (1,4-β-D-glucanglucanohydrolase, EC 3.2.1.4) breaks non-covalent interactions between linear cellulose chains and links of internal β-1,4-glycosidic leaving more free short cellulose chains, alters the crystalline structure of cellulose and exposes the polysaccharide chains of individual cellulose. Exoglucanase (1,4-β-D-glucan-cellobiohydrolase, EC 3.2.1.91) forms from two or four glucose units from the ends of the exposed chains produced by endoglucanase and releases celotetrosa or cellobiose from non-crystalline cellulose. Finally, β-glucosidase or cellobiase (β-D-glucoside glucohydrolase, EC 3.2.1.21) hydrolyses cellobiose units and short oligosaccharide, obtaining individual monosaccharide glucose (Martins et al. , 2008; Lynd et al. , 2002).
The action of cellulases may be used for the transformation of certain substrates of agroindustrial interest, considering the high content of cellulose, as in the case of oil palm empty fruit bunches (Eleaeis guinensis Jacq.) (Alam et al. , 2009; Umikalsom et al. , 1997). This substrate is the main solid byproduct of the oil production process, which is of great importance within agribusiness in Colombia, Malaysia, Indonesia, Thailand and Nigeria (García-Núñez et al. , 2008). The empty fruit bunch correspond to 23% of the weight of fresh fruit bunches processed into oil. Just one oil production plant with a capacity of 60 t of fruit bunches per hour produces more than 54,000 t of empty fruit bunches per year, with reported cellulose content between 37 and 62% (Chew and Bhatia, 2008). In Colombia, contents of cellulose and hemicellulose in empty fruit bunches have been reported at 47 and 21% respectively, with 41% C, 0.87% N and 0.09% S, and although some empty fruit bunches are used in boilers for steam production, a plant typically has 30% of the total biomass available for other uses (García, 1993). Due to the heat sterilization process and the temperature to which the clusters are subjected for oil extraction, microorganisms such as enzymes involved in the natural decomposition process of empty fruit bunches are denatured, increasing decay time in the field, causing severe environmental and health problems (Atlas, 2002; Prasertsan and Prasertsan, 1996). Additionally, in many producing areas, environmental authorities increasingly restrict the direct disposal of empty fruit bunches in the field.
Cellulytic activity has been reported on in vitro for empty fruit bunches of A. niger (Prasertsan and Prasertsan, 1996; Wen et al. , 2005), Trichoderma reesei (Rodríguez and Piñeros, 2007; Wen et al. , 2005) and Chaetomium sp. (Umikalsom et al. , 1997), among others. However, none of the strains, including the best mutant, are capable of producing high concentrations of the three types of enzymes at the same time (Zhou et al. , 2008). For example, T. reesei produced CBH and EGs in high quantities, but β-glucosidase activity was low in contrast to A. niger which produces more β-glucosidase but is limited for EG (Dashtban et al. , 2009) An alternative to supplement this deficiency is the use of enzymes for hydrolysis of vegetable biomass, either purified or in a culture of supernatants of cellulolytic fungi species, with an exogenous addition which depends on the purpose of the transformation and may be enhanced by glucosidases supplementation and commercial xylanases (Kovacs et al. , 2009).
As part of the activities the Colombian Center for Genomics and Bioinformatics of Extreme Environments (GeBix), the collection and characterization of filamentous fungi in different ecosystems of the high andean forest of the Parque Nacional Natural de los Nevados was carried out (PNNN) (Avellaneda et al. , 2009) in order to determine the feasibility of their use in biotechnological processes, in which isolates were obtained in vitro with high cellulolytic capacity, determined by activity curves for endoglucanase, exoglucanase, and β-glucosidase. The most promising strains, designated B7, B11, B11M and B19, along with two isolates obtained from palm empty fruit bunch decay (Moya-álvarez, 2012), denominated TA1 and TA2, were characterized by activity curves for endoglucanase, glucosidase and β-exoglucanase, in a Mandel's medium with cellulose and empty fruit bunches as carbon sources. The most promising isolate corresponds to the strain B19 (Penicillium sp.), having an endoglucanase activity of 277.88 nkat mL-1 and exoglucanase activity of 88.18 nkat mL-1 at 300 h of incubation in cellulose. Likewise, it was found that the strain B7 has a potential β-glucosidase activity of 25.5 and 17.17 nkat mL-1, obtained in cellulose and empty fruit bunches, respectively (Moya-álvarez, 2012).
As a consequence of the information obtained from the isolates, we conducted the present study, which aimed to determine the ability of hydrolysis of empty fruit bunches and microcrystalline cellulose and the presence of an enzymatic synergy in vitro of the enzyme consortia obtained from the mixture of supernatants individually produced from promising fungi of the PNNN soil and decaying palm empty fruit bunches of the eastern plains of Colombia.
Materials and methods
Fungal strain
The study employed four isolates of filamentous fungi from high-andean forests of the PNNN with a high activity of total cellulase, endoglucanase, exoglucanase and β-glucosidase, in accordance with a previous characterization performed at the Agro-biotechnology lab, Universidad Nacional, called B-7, B-11, B-11M and B-19, corresponding to Penicillium sp. (Avellaneda et al. , 2009); and two isolates from decaying palm empty fruit bunches called TA1 and TA2 and identified as Emericella nidulans strain EN-KSU-09 and Aspergillus fumigatus, respectively (Moya-álvarez, 2012). The initial inoculum was obtained from agar plates with CMC as a carbon source, incubated until the appearance of conidia at 25°C, suspended in water with 1% (v/v) Tween® 80, and homogenized at a concentration of 2·106 spores/mL.
Culture medium
A modified Mandels medium was used, with the following composition: 0.004 g L-1 NH4Cl, 2.1 g L-1 (NH4)2SO4, 2.0 g L-1 KH2P2O4, 0.3 g L-1 CaCl2 , 0.3 g L-1 MgSO4 7H2O, 0.00156 g L-1 MnSO4 5H2O, 0.0014 g L-1 ZnSO4 7H2O, 0.00266 g L-1 CoCl2 6H2O, 0.25 g L-1 yeast extract, 0.01 g L-1 succinic acid, 10 g L-1 microcrystalline cellulose powder (20 µ) (St. Louis, MO) or empty fruit bunches pretreated with 0.5 M NaOH and 0.5% (v/v) H2O2, 29.94 g L-1 with a content of 55.5% (w/v) cellulose, 20% (w/v) hemicellulose, and 18.2% (w/v) lignin (Moya- Álvarez, 2012). The pH was adjusted to 5.0 before sterilization (Kumar, 2001; Umikalsom et al. , 1997). Supernatants with cellulase activity used for hydrolysis were obtained in 15 mL tubes with 5 mL of culture medium and incubated at 25°C for 300 h with orbital shaking at 130 rpm. The consortia were prepared with a 1:1 mixture of the individual supernatants.
Determination of endoglucanase, exoglucansa and β-glucosidase activity
Before determining the hydrolysis of the supernatants, cellulolytic activity was determined. For endoglucanase activity, the study employed a 50 µL substrate of carboxymethyl cellulose (CMC), (Sigma, St. Louis, MO) 0.8% (w/v) in a sodium acetate buffer pH 5.0 with 50 µL of supernatant from each fungus, according to Ghose (1987). For the exoglucanase determination, a substrate was used of 15 µL of p-nitrophenyl-β-D-cellobioside (pNPC) (Sigma, St. Louis, MO) at 1% (w/v) as a substrate under the same conditions and 100 µL of supernatant. Both reactions were incubated at 40oC for 1 h and 4°C for 10 min. Then, 50 µL of the solution was mixed with 50 µL of a DNS solution (Ghose 1987), 1% (w/v) NaOH and 16% (w/v) sodium potassium tartrate, 43.8% (v/v) distilled water, for determination of released reducing sugars. The mixture was incubated for 5 min at 90°C and then cooled 10 min at 4°C. Then, 50 µL of the above solution were added with 250 µL of distilled water. Absorbance readings were made at 540 nm in a microplate reader model BioRad 680XR (Bio- Rad Laboratories, Hercules, CA) from the Biotechnology Laboratory, Faculty of Agronomy, Universidad Nacional de Colombia. Calibration curves were made with glucose (0.1, 0.7, 1.5, 2.5 and 3 g L-1) for endo-and exoglucanases taking into account that one unit of enzymatic activity corresponds to 1 µmol min-1 of glucose released during hydrolysis.
Determination of β-glucosidase activity
We used 180 µL of ρ-nitrophenyl-β-D-glicopiranósido (PNPG) (Sigma, St. Louis, MO) as a substrate and 50 µL of supernatant. Following incubation with the substrate, 20 µL of this solution were added with 80 µL of 2% sodium carbonate and 180 µL water. We measured the release of p-nitrophenol as the increase in absorbance at 405 nm and used pNitrofenol (10, 20, 30 and 50 µg mL-1) for the calibration curve of β-glucosidases (Sadana and Patil, 1988). In all cases, the tests were performed in triplicate.
Determination of hydrolysis of supernatant consortia
The fungal cellulases for the study were obtained from 15 mL Falcon tubes with 4 mL of modified Mandel's medium with cellulose (Umikalsom et al. , 1997) as the carbon source, incubated under stirring at 130 rpm and 25°C for 300 h. Each culture was centrifuged at 4.500 gn for 15 min. The supernatant corresponded to the crude enzyme extract. Enzymatic hydrolysis was performed in triplicate in 1.5 mL tubes with 100 µL of acetate buffer and 100 µL of cell-free supernatants containing the enzyme, with 1% (w/v) microcrystalline cellulose and empty fruit bunches 5% (w/v) as the substrate at 45°C and 175 rpm on an orbital shaker MaxQ 4000 (Barnstead/Labline, Melrose Park, IL) for 2 h. The total volume of enzyme extract was 100 µL, and was divided according to the number of strains in each mixture. Thus, for the mixture of supernatants obtained from two strains each volume was 50 µL and for mixtures of four strains, it was 25 µL. A control with acetate buffer pH 5.0 was used. The glucose obtained was determined at 2 h of incubation with the DNS method (Ghose, 1987). A factorial model was used with carbon source as the main factor and strain as a secondary factor.
Determination of synergy between the enzymes of the consortium
The determination of the synergy between the enzymes when acting in consortium was performed as reported by Andersen et al. (2008). Wherein, a measure of synergy can be calculated as the ratio of the activity exhibited by a mixture of components divided by the sum of activities of the components separately and which may be obtained based on the product formation, or the extent of substrate conversion. This measure is called the degree of synergy (DS). When the DS is greater than 1, there is said to be synergy between the components of the consortium, and competition when it is less than one.
Statistical analysis
Statistical analysis of the data was performed using a factorial model with carbon source, as the main factor, at two levels: cellulose or empty fruit bunches called "tusa". The secondary factor was strain with different levels according to the strains or consortia supernatants used. The evaluated variables correspond to protein biomass, endoglucanase, exoglucansa and β-glucosidase activity. Then Tukey and Sheff method with a significance level of P = 0.05 and 0.01 were used to detect statistically significant differences among of variable used. Each experiment was done in triplicate and the analysis of variances was conducted using SAS (v. 9.1) statistical software.
Results and discussion
Determining the hydrolyzing ability of the enzyme consortia
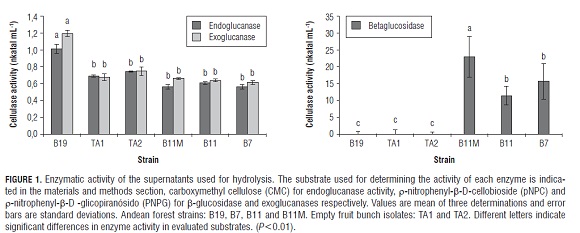
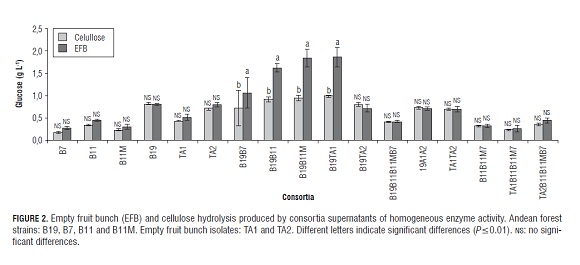
The supernatants used for forming consortia were individually characterized by endoglucanase, exoglucanase, and β-glucosidase activity. The cellulase activity of the supernatants used for hydrolysis is seen in Fig.1. The results obtained from hydrolysis with the supernatants obtained from the individual strains and their mixtures (consortia) are presented in Fig.2. B19 was observed with the greatest hydrolysis compared with supernatants produced by the individual strains. Given this fact, the hydrolysis of B19 was used as a comparison with that produced by the consortia and assumed to produce 100% hydrolysis.
Highly significant differences were observed in the microcrystalline cellulose hydrolysis due to the interaction consortium x substrate (P=0.01). In the consortia that showed significant differences, most hydrolysis occurred in empty fruit bunches with less crystallinity than microcrystalline cellulose and possibly a lower degree of polymerization. In this regard, it has been reported that the microcrystalline cellulose is degraded more slowly to cellobiose and glucose than amorphous cellulose, which is more accessible to enzymatic action (Yang et al. , 2011).
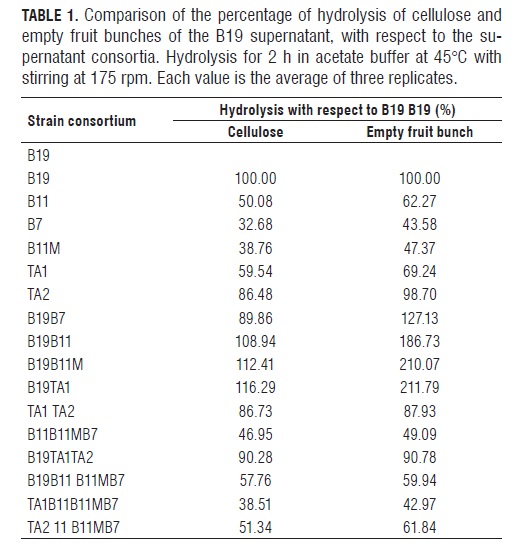
In empty fruit bunches, the consortia of B19B11, B19B11M and B19TA1 produced higher hydrolysis as compared to that of B19 individually at 86, 110 and 111%, respectively, as presented in Tab.1. There may be a synergistic effect between the supernatants mentioned. The hydrolysis of the more efficient consortia was close to that shown by the supernatant of B19 with 3.5 times greater enzymatic activity and was obtained from the individual supernatant of B19 at 300 h of growth, according to preliminary studies (Moya-álvarez, 2012). A similar effect was reported by Jørgensen et al. (2005) who, with increased β-glucosidase activity, obtained a saccharification of the substrate similar to that obtained with a lower activity.
According to the results, consortia culture supernatants produced individually could reduce empty fruit bunch hydrolysis costs considering that supernatants are obtained with lower enzyme activity, requiring less time of incubation with the consequent reduction in consumption energy.
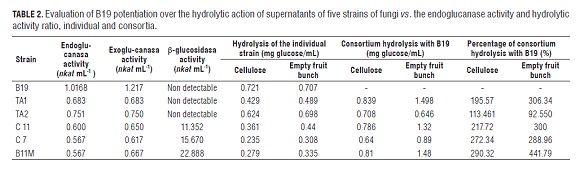
When comparing the hydrolysis of each individual supernatant with that obtained when in consortium with B19 (Tab.2), it was observed in empty fruit bunches, that the consortium hydrolysis of the B19 supernatant with TA1 was 306% greater than that obtained individually for TA1. For B11M, hydrolysis with B19 was 441% higher compared to B11M individually. In cellulose, although in all cases hydrolysis increased, the rate of increase was smaller; only in the TA2 consortium was hydrolysis higher in cellulose than empty fruit bunches.
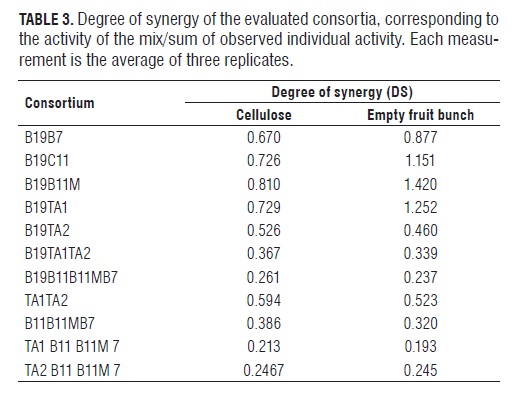
With the information obtained from the hydrolysis of the individual strains and consortia, the degree of synergy was calculated for the consortia based on the glucose concentration obtained as a product of the hydrolysis of cellulose and empty fruit bunches by supernatants corresponding to a mixture of the enzymes endoglucanase, exoglucanase, and β-glucosidase produced by each strain (Tab.3). It was determined that consortium synergy occurs only in empty fruit bunches in the consortia B19B11, B19B11M and B19TA1 with DS values greater than 1. In other consortia, there was competition, both for empty fruit bunches and cellulose, as the DS was less than 1. Interestingly, there was synergy between B19, the strain with the most endoglucanase and exoglucanase activity, and B11M and B11, the strains with the most β-glucosidase activity, as compared with TA1 and TA2. In these consortia, endoglucanase and exoglucanase initiated hydrolysis and β-glucosidase hydrolyzed cellobiose produced by the two primaries to produce glucose, as described in the literature as one of the possible action mechanisms of the cellulase enzyme complex. This effect has been observed in enzyme consortia of A. niger with T. reesei, the latter deficient in β-glucosidase, essential to convert cellobiose to glucose (Alam et al. , 2005). The synergy of endoglucanases and exoglucanases with β-glucosidase has been described in the enzyme interaction of P. pinophilum and T. koningii (Wood and McCrae, 1986).
The results obtained in this study do not agree with the claim of Andersen (2008) that as a rule the highest DS occurs in highly crystalline substrates (such as crystalline cellulose and cotton) and decreases with the decrease of the crystallinity of the substrate. In this case, the higher obtained DSs were in empty fruit bunch consortia, a substrate with crystallinity lower than microcrystalline cellulose, where DSs were lower.
The hydrolysis produced by B19, 0.721 and 0.707 g L-1, respectively for microcrystalline cellulose and empty fruit bunches, with supernatants with 5.001 nkat mL-1 of endoglucanase activity and nkat mL-1 of exoglucanase activity was similar to that obtained by Ariffin et al. (2008) with 0.73 g L-1 of reducing sugars in untreated empty fruit bunches using a pure enzyme that contained 941.85 nkat mL-1 total cellulase, 2458.825 nkat mL-1 endoglucanase, and 280.05 nkat mL-1 β-glucosidase. Considering the difference in the activity of enzymes used in this study, one can conclude that the B19 supernatants used in this study are highly efficient. The subsequent evaluation of pretreatments is also important, increasing availability of cellulose since the synergy could be modified with actions over the availability of cellulose in empty fruit bunches with pretreatments that reduce the concentration of lignin and hemicellulose as has been previously demonstrated (Simarani et al. , 2009).
In this study, it was determined that the consortia B19B11, B19B11M and B19TA1 obtained 1.5 g of reducing sugars per g empty fruit bunches 5% (W/V) and 0.8 g g-1 of cellulose 5% (W/V) with supernatants for B19 with 1.02, 0.061, and 1.22 nkat mL-1 of endoglucanase and exoglucanase activity, respectively, 0.04 and 0.67 nkat mL-1 for the other strains (Tab.2). This result agrees with those previously obtained by Umikalsom et al. (1997) who obtained 0.7 g reducing sugars per g of delignified empty fruit bunches (78% cellulose and 5.5% lignin) with enzymes of Chaetomium globosum with 250 nkat mL-1 PFasa, 7134.7 nkat mL-1 , CMCase (endoglucanase) and 1500 nkat mL-1 β-glucosidase. The supernatant of B19, acting individually, produced 0.75 g of reducing sugars for both empty fruit bunches and cellulose. Other authors reported that an endophytic fungus isolated from the paramo of Cruz Verde (Cundinamarca) was able to produce more than 25 g L-1 of reducing sugars in 6 d with chemically pretreated empty fruit bunches as the carbon source, possibly due to the action of endo and exoglucanases (González et al. , 2008). These data, however, are not comparable with those obtained by the strains under the present study; the evaluation was performed at 2 h of incubation and cannot be extrapolated to 6 d since the linearity of the reaction is not known. In general, one could say that the time of empty fruit bunch digestibility is enhanced by a reduction in crystallinity index of cellulose and an increase in surface area (Park et al. , 2010). Likewise, the evaluation would be needed over time, where factors of stability of the supernatant enzyme mixture would be related. It is possible that the consortia that effectively increased empty fruit bunch digestion contributed to the reduction of surface roughness of the crystalline cellulose with the increased traffic of cellulases, flattening the surface, removing obstacles and increasing the number of inputs and outputs of enzymes which reduce congestion, improve mobility of cellulases and increase the efficiency of the hydrolysis (Igarashi et al. , 2011).
Conclusions
The study allowed us to determine the ability of empty fruit bunch and cellulose hydrolysis, in vitro, by consortia of enzymes obtained from the mixture of supernatants produced by fungi from a high andean forest transect of the Parque Natural Nacional de Los Nevados and decaying empty fruit bunches of individually cultivated oil palms. The efficiency of the hydrolysis of empty fruit bunches by supernatants of consortia of B11, B11M and TA1 with B19 is greater than that obtained by the supernatants produced by each strain individually, with hydrolysis capacity increases up to 400%. The action of such consortia could be complemented by the timely addition of supernatants of B7 due to the β-glucosidase activity. The dilution of the activity of the supernatant of B19, 5.001 to 1.002 nkat mL-1 of endoglucanase activity, does not reduce the capacity of hydrolysis in consortia.
Promising consortia here show great potential and the action can be optimized with the study of the characteristics of the enzymes involved and their interactions. Thus, it would be possible to obtain a balanced synergy that takes into account: suitable combinations of enzymes, which maximize hydrolysis, achieve higher performance and reduced glucose production costs. It is necessary to take into account aspects such as the proportion of supernatants of each strain of the consortium, time in which they are added, pH, agitation, reaction temperature and reaction medium. It is also important to note that to completely disassemble heterogeneous structures of plant cell walls, reactions also require synergy with other enzymes such as hemicellulases and ancillary enzymes and modifying lignin (Yang et al. , 2011).
The use of consortia of supernatants from cultivations of the studied fungi has great potential for the initial treatment of empty fruit bunches after oil extraction, considering that the temperature after the sterilization process in the processing plant may be optimal for the action of cellulases. Thus, hydrolysis to glucose could be initiated more rapidly in empty fruit bunches, which could allow a faster colonization by decomposing microorganisms with an easily employed increase in substrate concentration and could therefore reduce decomposition time, which should be further tested. On the other hand, subsequent studies could be directed towards optimizing hydrolysis to glucose with different purposes.
Acknowledgements
The authors wish to thank the Colombian Center for Genomics and Bioinformatics of Extreme Environments (GeBix), Colciencias. Unipalma plantation, and the Universidad Nacional de Colombia for financial support; the Laboratory of biotechnology, Faculty of Agronomy, Universidad Nacional de Colombia, particularly Rosa Mejía for management of the laboratory activities; and the Plant Biotechnology Laboratory, Faculty of Agronomy for the use of equipment for enzymatic determinations.
Literature cited
Alam, M.Z., A.A. Kabbashi, S. Nahdatul, and I.S. Hussin. 2009. Production of bioethanol by direct bioconversion of oil-palm industrial effluent in a stirred-tank bioreactor. J. Ind. Microbiol. Biotechno. 36, 801-808. [ Links ]
Alam, M.Z., N. Muhammad, and M. Mahmat. 2005. Production of cellulase from oil palm biomass as substrate by solid state bioconversion. American Journal of Applied Sciences. [ Links ]
Andersen, N., K. Johansen, M. Michelsen, E. Stenby, M. Krogh, and L. Olsson. 2008. Hydrolysis of cellulose using mono-component enzymes shows sinergy during hydrolysis of phosphoric acid swollen cellulose (PASC), but competition on Avicel. Enz. Microb. Technol. 42, 362-370. [ Links ]
Ariffin, H., M. Hassan, M.S. Umi Kalsom, N. Abdullah, F. Ghazali, and Y. Shirai. 2008. Production of bacterial endoglucanase from pretreated oil palm empty fruit bunch by Bacillus pumilus EB3. J. Biosci. Bioeng. 106(3), 231-236. [ Links ]
Atlas, R. 2002. Ecología microbiana y microbiología ambiental 4th ed. Adisson Wesley, Madrid. [ Links ]
Avellaneda, M.L., C.P. Guevara, R. Mejía, and E. Torres 2009. Determinación de la actividad celulolítica de microorganismos presentes en suelos del Parque Nacional Natural de Los Nevados, Colombia. p. 67. In: Memorias Primer Congreso Iberoamericano de Química, Bioquímica and VII Congreso Internacional de Química y Biología Molecular. La Habana. [ Links ]
Chew, T. and S. Bhatia. 2008. Catalytic processes towards the production of biofuels in a palm oil and oil palm biomass-based biorefinery. Biores. Technol. 99, 7911-7922. [ Links ]
Dashtban, M., H. Schraft, and W. Qin. 2009. Fungal bioconversion of lignocellulosic residues; opportunities & perspectives. Rev. Intl. J. Biol. Sci. 5(6), 578-595. [ Links ]
García, J. 1993. Estado actual del manejo de efluentes en Colombia. Rev. Palmas 14 (Special issue), 141-148. [ Links ]
García-Núñez, J.A., M. Garcia-Pérez, and K.C. Das. 2008. Determination of kinetic parameters of thermal degradation of palm oil mill by products using thermogravimetric analysis and differential scanning calorimetry. Trans. ASABE 51(2), 547-557. [ Links ]
Ghose, V.K. 1987. Measurement of cellulase activities. Pure Appl. Chem. 59, 257-268. [ Links ]
González, A., I. Jiménez, M. Susa, S. Restrepo, and J. Gómez. 2008. Biocombustibles de segunda generación y biodiesel: una mirada a la contribución de la Universidad de Los Andes. Rev. Ing. 28, 70-82. [ Links ]
Igarashi, K., T. Uchihashi, A. Koivula, M. Wasa, S. Kimura, T. Okamoto, M. Penttila, T. And, and M. Samejima. 2011. Traffic jams reduce hydrolytic efficiency of cellulase on cellulose surface. Sience 333(6047), 1279-1282. [ Links ]
Jørgensen, H., A. Mørkeberg, K.B.R. Krogh, and L. Olsson. 2005. Production of cellulases and hemicellulases by three Penicillium species: effect of substrate and evaluation of cellulase adsorption by capillary electrophoresis. Enz. Microb. Technol. 36, 42-48. [ Links ]
Kovacs, K., S. Macrelli, G. Szakacs, and G. Zacchi. 2009. Enzymatic hydrolysis of steam-pretreated lignocellulosic materials with Trichoderma atroviride enzymes produced in-house. Biotechnol. Biofuels 2, 1-14. [ Links ]
Kumar, R.S. 2001. Semi-solid-state fermentation of Eicchornia crassipes biomass as lignocellulosic biopolymer for cellulase and ß-glucosidase production by cocultivation of Aspergillus niger RK3 and MTCC164. Appl. Biochem. 96(1-3), 71-82. [ Links ]
Lynd, L.R., P. Wimer, W.H. Van Zyl, and I.S. Pretorius. 2002. Microbial cellulose utilization: fundamentals and biotechnology. Microb. Mol. Biol. Rev. 66(3), 506-577. [ Links ]
Martins, L., D. Kolling, M. Carnassola, A. Pinheiro-Dillan, and L. Pereira-Ramos. 2008. Comparison of Penicillium echinulatum and Trichoderma reesei cellulases in relation to their activity against various cellulosic substrates. Bioresource Technol. 99, 1417-1424. [ Links ]
Moya-Álvarez, L. 2012. Determinación de la capacidad celulolítica de consorcios de hongos provenientes de bosque Altoandino in vitro en tusas de palma de aceite. M.Sc. thesis. Faculty of Sci, Universidad Militar Nueva Granada, Bogota. [ Links ]
Rodríguez G., I. and Y. Pineros. 2007. Producción de complejos enzimáticos celulolíticos mediante el cultivo en fase sólida de Trichoderma sp. sobre los racimos vacíos de palma de aceite como sustrato. Vitae 14(2), 35-42. [ Links ]
Park, S., J. Baker, M. Himmel, P. Parilla, and D. Johnson. 2010. Celulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 3, 1-10. [ Links ]
Prasertsan, S. and P. Prasertsan. 1996. Biomass residues from palm oil mills in thailand: an overview on quantity and potential usage. Biom. Bioene. 11(5), 387-395. [ Links ]
Sadana, J.C. and R.V. Patil. 1988. 1,4-ß-D-Glucan cellobiohydrolase from Sclerotium rolfsii. Meth. Enzymol. 160, 307-314. [ Links ]
Simarani, K., M. Hassan, S. Abd-Aziz, and M.S. Wakisaka. 2009. Effect of palm oil mil sterilization process on the physicochemical characteristics and enzymatic hydrolysis of empty fruit bunch. Asian J. Biotechnol. 1(2), 57-66. [ Links ]
Singh, P., O. Sulaiman, and R. Hashim. 2010. Biopulping of lignocellulosic material using different fungal species: a review. Rev. Environ. Sci. Biotechnol. 9, 141-151. [ Links ]
Umikalsom, M.S., A. Ariff, H. Zulkifli, C. Tong, M. Hassan, and M. Karim. 1997. The treatment of oil palm empty fruti bunch fibre for subsequent use as substrate for cellulase production by Chaetomium globosum Kunze. Bioresource Technol. 62, 1-9. [ Links ]
Wen, Z., W. Liao, and S. Chen. 2005. Production of cellulase/ß- glucosidase by the mixed fungi culture Trichoderma reesei and Aspergillus phoenicis on dairy manure. Process Biochem. 40, 3087-3094. [ Links ]
Wood, T. and S.I. McCrae. 1986. The cellulase of Penicillium pinopilum Synergism between enzyme components in solubilizing cellulose with special referencie to the involvment of two inmunologically distinct cellobiohydrolases. Biochem. J. 234, 93-99. [ Links ]
Yang, B., Z. Dai, S.-Y. Ding, and C. Wyman. 2011. Enzymatic hydrolysis of cellulosic biomass. Biofuels 2(4), 421-450. [ Links ]
Zhou, J., S.-L. Si-Liang Zhang, and P. Yin. 2008. Identification and purification of the main components of cellulases from a mutant strain of Trichoderma viride T 100-14. Bioresource Technol. 99, 6826-6833. [ Links ]