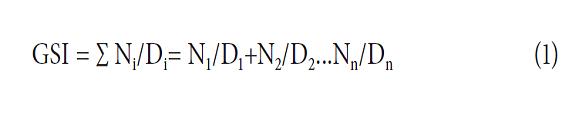Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Agronomía Colombiana
Print version ISSN 0120-9965
Agron. colomb. vol.31 no.1 Bogotá Jan./Apr. 2013
PROPAGATION & TISSUE CULTURE
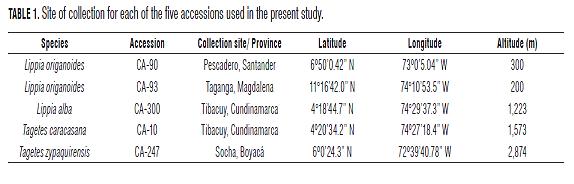
Establishment of propagation methods for growing promising aromatic plant species of the Lippia (Verbenaceae) and Tagetes (Asteraceae) genera in Colombia
Establecimiento de métodos de propagación para el cultivo de especies vegetales aromáticas promisorias en Colombia de los géneros Lippia (Verbenaceae) y Tagetes (Asteraceae)
Axel Mauricio Herrera-Moreno1, Carlos Edwin Carranza1 and María Isabel Chacón-Sánchez 1
1Department of Agronomy, Faculty of Agronomy, Universidad Nacional de Colombia. Bogota (Colombia). michacons@unal.edu.coReceived for publication: 7 May, 2012. Accepted for publication: 29 March, 2013.
ABSTRACT
The objective of this study was to evaluate methods of asexual propagation with stem cuttings in the aromatic plant species Lippia origanoides (accessions CA-90 and CA-93), Lippia alba (accession CA-300) and Tagetes zypaquirensis (accession CA-247) and determine the germination behavior of Tagetes caracasana (accession CA-10), in order to contribute to the establishment of these promising aromatics as potential new crops in Colombia. The factors evaluated were the substrate (fine coconut fiber (FCF); fine coconut fiber: coal slag 1:1 (FCFCS); fine coconut fiber: coal slag: river sand 1:1:1 (FCFCSRS ) and IBA hormone concentration (0, 2,000 and 4,000 mg L-1). Germination tests of seeds of the accession CA-10 were carried out in Petri dishes with 50 seeds per dish, with three replicates in controlled conditions and constant temperature (25°C), humidity (90%) and total darkness. In general, the accessions CA-90, CA-93, CA-300 and CA-247 showed better rooting percentage, root length, number of roots and root dry weight in the fine coconut fiber substrate and a higher number of roots with an exogenous application of 2,000 mg L-1 IBA. L. alba and T. zypaquirensis responded better than L. origanoides to the treatments. The latter species showed a relatively poor performance and may require more complex and improved propagation methods to obtain more satisfactory results. T. caracasana seeds had a relatively short germination time (less than three weeks) and relatively high germination under controlled laboratory and greenhouse conditions (70 and 60%, respectively); these percentages are high relative to wild species of the same genus, meaning this method of seed propagation is appropriate for this wild species.
Key words: indolebutyric acid (IBA), auxins, seed germination, vegetative propagation, cuttings.
RESUMEN
El objetivo del presente estudio fue evaluar métodos de propagación asexual por estacas en las especies aromáticas vegetales Lippia origanoides (accesiones CA-90 y CA-93), Lippia alba (accesión CA-300) y Tagetes zypaquirensis (accesión CA-247), y determinar el comportamiento de la germinación de Tagetes caracasana (accesión CA-10), a fin de contribuir al establecimiento de estas especies promisorias como nuevos cultivos potenciales en Colombia. Los factores evaluados fueron el sustrato (fibra de coco fina (FCF); fibra de coco fina: escoria de carbón 1:1 (FCFEC); fibra de coco fina: escoria de carbón: arena de río 1:1:1 (FCFECAR ) y la concentración de la hormona AI B (0, 2.000 y 4.000 mg L-1). Las pruebas de germinación de las semillas de la accesión CA-10 se llevaron a cabo en platos de Petri con 50 semillas por plato, con tres réplicas, en condiciones controladas y temperatura constante (25°C), humedad (90%) y oscuridad total. En general, las accesiones CA-90, CA-93, CA- 300 y CA-247 mostraron mejor enraizamiento, longitud de las raíces, número de raíces y peso seco de las raíces en el sustrato FCF y un mayor número de raíces con la aplicación exógena de 2.000 mg L-1 AI B. L. alba y T. zypaquirensis respondieron mejor que L. origanoides a los tratamientos. Esta última especie mostró un comportamiento relativamente bajo y puede requerir métodos de propagación más complejos y mejorados a fin de obtener resultados más satisfactorios. Las semillas de T. caracasana mostraron tiempos de germinación relativamente cortos (menor a tres semanas) y una germinación relativamente alta bajo condiciones controladas y en condiciones de invernadero (70 y 60%, respectivamente); estos porcentajes son relativamente altos con relación a especies silvestres del mismo género, indicando que la propagación por semilla es apropiada para esta especie silvestre.
Palabras clave: ácido indolbutírico (AI B), auxinas, germinación de las semillas, propagación vegetativa, estacas
Introduction
The cultivation of native plant species, with potential uses as spices, seasonings, and sources of essential oils (EO) and derived products in Colombia, is an excellent alternative for growers and has a potential application in agriculture (such as biological control) and industry. Colombia has unexplored flora with good potential for the production of EO. However, these plants, as is characteristic of native species that are often found in the wild, lack methods of propagation and agronomic management, which limits their use as potential new crops.
Furthermore, in Colombia, producers and processors of aromatic plant species face various limitations, including issues of quality, performance and traceability for processing. One consequence of this is the low participation of Colombia in the global market for aromatic plants. This low competitiveness is due to various factors, one of which is the lack of technology for mass propagation and crop management, and the lack of knowledge of the potential of native species that offer better alternatives for production and marketing.
The establishment of propagation and micropropagation techniques for aromatic species that show promise as potential new crops is important in order to ensure rapid reproduction to provide enough material for the high demand that can be generated in a production chain. One of the strategies in the development of a production chain is the establishment of mass production of seed and planting material, which requires standardization of propagation techniques.
Among the promising aromatic plants for Colombia based on quality and bioactivity of essential oils are species of the Verbenaceae family such as Lippia alba (Mill.) NE Br ex Britton & P. Wilson, known as "pronto alivio" (quick relief), and Lippia origanoides Kunth, known as "orégano de monte" (mountain oregano), and species of the Asteraceae family, of the Tagetes genus such as Tagetes zypaquirensis Bonpl. and Tagetes caracasana Humb. ex Willd., among others (Hernandez-Lozano et al., 2010; Meneses et al., 2009; Nerio et al., 2009; Pascual et al., 2001; Stashenko et al., 2010). The genus Tagetes is native to the New World and the species T. erecta (known as marigold) and T. patula are the main cultivars of this genus and were domesticated in pre-Columbian times (Kaplan, 1958). According to herbarium records of the Missouri Botanical Garden, L. alba is widely distributed throughout the Americas, while the last three species are reported in northern South America only. Accordingly, the species L. alba is one of the most studied within the Lippia genus, while the other three species are much less known.
For the Lippia genus, in vitro micropropagation protocols have been reported that manage the production of plants with chemical profiles identical to plants normally propagated (Gupta et al., 2001; Juliani et al., 1999). The reports indicate that the seeds of L. alba have low germination, therefore, for this species, asexual propagation by stem cuttings is preferred (Pimenta et al., 2007; Seaforth and Tikasingh, 2008). There is one study that suggests the use of seeds for propagation of several wild species of Lippia from Brazil where asexual propagation methods have proved inefficient (Pimenta et al., 2007). For some Lippia species that are in danger of extinction such as L. filifolia (Pereira Peixoto et al., 2010) and L. dulcis, promising for its hernandulcin content (Sauerwein et al., 1991), efficient micropropagation methods have been developed. L. origanoides has been the subject of very few studies; there are only a few reports on agronomic (Paternina, 2009) and ecophysiological aspects (Antolinez-Delgado and Rodríguez, 2008; Camargo and Rodríguez, 2008; Parra and Rodríguez, 2007).
For Tagetes, there are a few reports related to methods of sexual and asexual propagation (Ferreira et al., 2001; Hidalgo, 1995) and agronomic aspects (Mariotti et al., 2010; Rojas, 1994). Tagetes minuta is a species that can be propagated by seeds (achenes) with germination percentages between 50-60%, or between 62-83% depending on conditions, in seeds sown directly in the soil or germinated in glass Petri dishes (Drewes and Staden, 1990; Ferreira et al., 2001; Singh et al., 2003). For T. minuta, micropropagation methods have been developed for in vitro production of secondary compounds used for the pharmaceutical, agronomic and food industries (Mohamed et al., 1999). For T. erecta, plant regeneration systems from leaf explants have been developed as tools for genetic manipulation (Misra and Datta, 2001; Vanegas et al., 2002). Specifically for T. caracasana and T. zypaquirensis, there are no reports in the literature related to methods of propagation.
Establishing propagation methods for promising aromatic species is important because there may be some relationship between the propagation method and cultural practices used and the yield and composition of essential oils and other chemical compounds. For example, in Mexican oregano (Poliomintha glabrescens Gray), plants propagated by in vitro micropropagation methods showed an increase in luteolin compared to plants growing wild (García-Pérez et al., 2012). Musarurwa et al. (2012) studied the effect of nitrogen, potassium, water stress and phytohormones on the essential oils of Salvia stenophylla (Burch. ex Benth.) and concluded that an increase in macronutrients was related to an increase in some compounds such as (-) - alpha-bisabolol.
Taking into account these facts, the objective of this study was to contribute to the establishment of the aromatic species L. origanoides, L. alba, T. zypaquirensis and T. caracasana as potential new crops in Colombia, through the evaluation of propagation methods for each of these promising species, which were selected for their potential for essential oil production.
Materials and methods
Study species
Propagating material was taken from stock plants previously established in the greenhouses of the Facultad de Agronomía, Universidad Nacional de Colombia, Bogota. Based on previous observations on the yield and composition of essential oils and bioactivity tests on plant pathogens (unpublished data), five accessions of the four species under study were selected, namely, L. origanoides (accessions CA- 90 and CA- 93), T. zypaquirensis (accession CA-247), Lippia alba (accession CA-300) and T. caracasana (accession CA- 10). The collection site for each of the accessions is shown in Tab.1. Due to the low perceptibility and complexity of seed selection and management in these species, the propagation methods used for evaluation were vegetative (asexual reproduction), except for the species T. caracasana where seed propagation was used. Again, there are no propagation methods established for T. caracasana and T. zypaquirensis, but according to our observations, T. caracasana flowered regularly in the greenhouse and produced abundant achenes, which germinated relatively easily in the soil, and for this reason the propagation method selected for this species was by seed. In contrast, T. zypaquirensis, although it flowered regularly in the greenhouse, did not produce seeds, and for this reason the propagation methods evaluated for this species were asexual.
Geographic location and conditions of the experiment
The propagation and evaluation were carried out in greenhouse conditions at the facilities of the Universidad Nacional de Colombia, Bogota, located at 2,556 m a.s.l. at coordinates 4°38'08,46" N and 74°0.5'11.99" W. The temperature and relative humidity in the greenhouse ranged between 11.9 and 38.9°C, and 29 and 88%, respectively. Stock plants were established from cuttings collected in the field. Cuttings were planted in germination trays, covered with transparent plastic to maintain high humidity and irrigated with a micro spray system. After a period of eight weeks, the seedlings were transplanted in a greenhouse with plastic cover and grown during a period of at least 32 weeks, after which the plants accumulated enough biomass to provide cuttings. Cuttings were taken from flowering stock plants.
The cuttings were selected from the middle stratum of the plant and branches, with a size between 12 and 14 cm, 2 to 3 nodes, and semi-woody texture. The cuttings were immersed for 5 min in liquid solution at different concentrations of the hormone indole-3-butyric acid (IBA) and were subsequently planted in germination trays with 24 wells, with dimensions of 5.8 x 5.8 x 8.1 cm, and covered with transparent plastic (to maintain humidity). In previous studies, it has been observed that an immersion time of 5 min at different IBA concentrations is necessary to promote rooting in cuttings of other plant species (Frangi and Nicola, 2005; Struve and Moser, 1984). The trays with the cuttings were distributed on a raised greenhouse bed and moistened with a micro spray system with micro sprinklers (40 L h-1) spaced every 30 cm, using a run time of 5 min d-1.
Experimental design and variables measured
The experiment was conducted with a completely randomized design and a 3 x 3 factorial arrangement (factors: substrate and IBA concentration) with three replicates for a total of nine treatments. The three substrates tested were fine coconut fiber (FCF), fine coconut fiber: coal slag (1:1 V:V) (FCFCS) and fine coconut fiber: coal slag: river sand (1:1:1 V:V:V) (FCFCSRS ). The three levels of IBA (99%) were 0 mg L-1, 2,000 mg L-1 and 4,000 mg L-1. The experimental unit was composed of ten cuttings, for an experiment size of 270 cuttings for each accession.
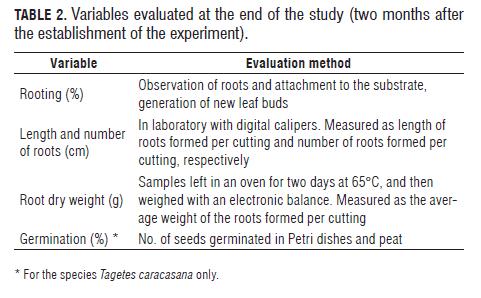
Upon completion of two months in the study, three cuttings were sampled at random for each experimental unit, for a total of nine cuttings evaluated per treatment; size was subject to the availability of viable material in response to each treatment and species, after the mentioned period. The variables evaluated at the end of two months for the four accessions are summarized in Tab.2.
In the case of the species T. caracasana, 50 seeds (raw seeds were collected by hand, after which there was a selection process in which defected seeds were discarded) were tested for germination in a Petri dish with three replicates (one Petri dish per repetition) arranged in a germination chamber (Seedburo Equipment) under controlled conditions and constant temperature (25°C), humidity (90%) and total darkness during the time of the test (Besnier, 1989). The same test was performed in germination trays with peat TRM7, with two replicates of 50 seeds, arranged in a greenhouse. Measurements were taken every week from germinated seeds (in this study, germination was defined as radicle protrusion, according to Drewes and van Staden, 1990). The variables evaluated were the percentage of germination (PG) and germination speed index (GSI ). The latter variable determines the number of seeds germinated at intervals of seven d and its numerical value was estimated according to Moreno et al. (2006):
where Ni is the number of germinated seeds (radicle protrusion) and Di is the number of days from sowing (observations were made every seven d).
Seeds for germination tests were harvested two d before the tests from 4 month-old stock plants. Stock plants were established from seeds collected from mature plants growing wild. Seeds brought from the wild were sown on a raised greenhouse mist bed where germination took place in a period of about three weeks. Seedlings were then transplanted to the field two months later to establish stock plants.
Statistical analyses
Initially, descriptive statistics were estimated, and then experimental design assumptions regarding normality, validity, representativeness (Levene and/or Bartlett test) and homogeneity of variances (Kolmogorov-Smirnov) were validated. An analysis of variance (ANOVA) and multiple comparison tests to evaluate differences between the effects of the treatments were used. Data analyses were performed independently for each of the four accessions asexually propagated using the SAS ® statistical package v. 9.0 (Statistical Analysis System Institute Inc, Cary, NC) and the use of Excel® 2011 for the sexually propagated accession.
Results and discussion
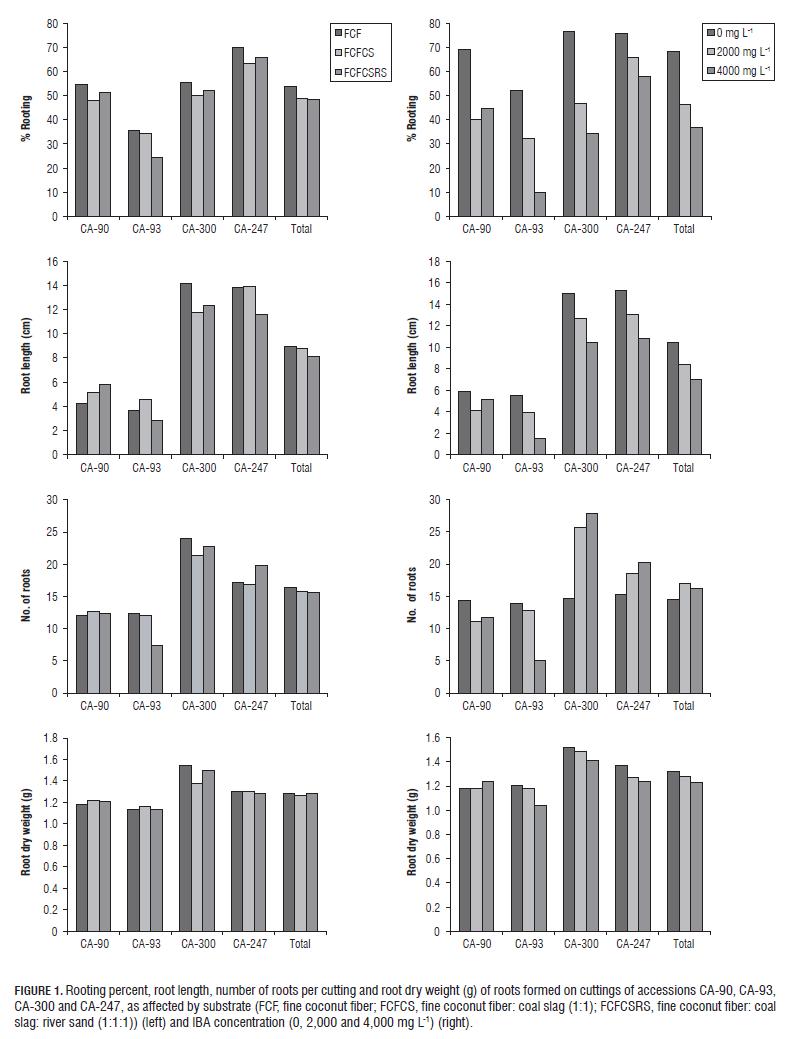
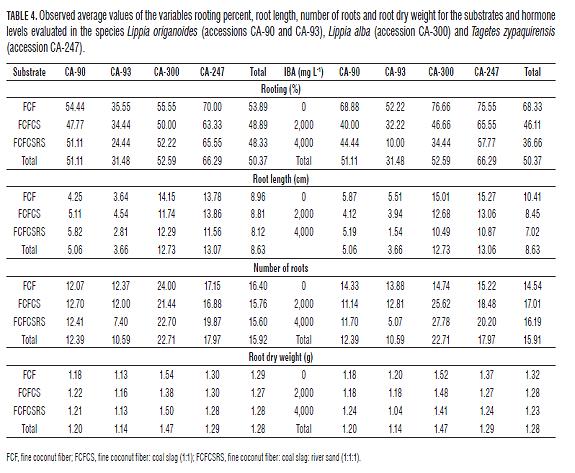
The Tab.3, Tab.4 and Fig.1 show the results of the multiple comparison tests and the observed average values of the variables rooting percentage, root length, number of roots and root dry weight for the substrates and hormone levels evaluated in the three species: L. origanoides (CA-90 and CA-93), L. alba (CA-300) and T. zypaquirensis (CA-247). The results for each species are presented below.
Genus Lippia (Verbenaceae)
Lippia origanoides
Accession CA-90. It can be observed in Tab.3 that for this accession, multiple comparison tests showed significant differences in root length for the different substrates, and significant differences in all the variables for the different concentrations of the IBA hormone. In this accession, cuttings propagated in the absence of the exogenous hormone showed generally higher rooting percentages (57 to 83%) than those propagated in the presence of the hormone (between 33 and 53%). Also, in the absence of hormone, cuttings showed longer roots and larger number of roots (Fig.1). The highest percentage of rooting for cuttings propagated in the absence of the hormone was achieved with the substrate FCF (83%), while longer roots (6,81 cm) were observed in cuttings propagated in the substrate FCFCSRS and hormone absence. On the other hand, root dry weight was not affected by substrate or IBA concentration.
The results of this study are similar to other reports in other plant species such as the Atlantic white cedar (Chamaecyparis thyoides [L.] B.S.P.) and the paperbark maple (Acer griseum) where the hormone IBA showed no effect on the rooting percent of stem cuttings (Hinesley et al., 1994; Maynard and Bassuk, 1990). The higher percentage of rooting observed in this study in the absence of exogenous hormone could be due to the accumulation of endogenous auxins that promote root initiation (Casimiro et al., 2003). Accumulation of endogenous auxins may be due to two major factors: a localized formation of auxin or the blockage of exogenous auxin transport/flux. Certain flavonoids have been found to act as auxin transport inhibitors that participate in regulating auxin flow in plant tissues (Wasson et al., 2006; Peer and Murphy, 2007).
In summary, both the substrate fine coconut fiber and the non-application of IBA (Tab.3, Fig.1) affected the rooting and the emission of roots in the cuttings of accession CA-90, maybe due to increased porosity of the substrate that generates more aeration for the establishment of the cuttings in this accession. Additionally, further observations showed significant positive effects of the FCFCSRS treatment in the absence of IBA on root length (Tab.3). The combination of substrates (FCFCSRS ) may generate a greater retention of moisture, favoring the emission of roots in the cuttings but not the weight of the roots (dry weight) formed per cutting. Black and Zimmerman (2002) proposed that inorganic substrates with coal slag could be suitable for rooting cuttings. Coconut fiber is a substrate with good buffering and high water retention capacities, which are useful for reducing the frequency of irrigation; and has a low bulk density (Quintero et al., 2011). Apparently, the combination of the three substrates generates physicochemical characteristics that help in rooting stem cuttings in this accession.
Accession CA-93. Tab.3 and Fig.1 show that for this accession, similar to accession CA-90, there were significant differences in root length for cuttings established in the different substrates. The rooting percentage and the number of roots were slightly higher in the substrate FCF, while root length and root dry weight performed better in the FCFCS substrate (Tab.4, Fig.1). For IBA concentration, there were significant differences for the variables rooting, root length and root dry weight for the different concentrations tested (Tab.3). In this accession, cuttings propagated in the absence of the hormone showed a generally higher rooting percentage (from 43 to 67%) than those propagated in the presence of the hormone (between 3 and 43%), and also showed longer roots and larger number of roots and root dry weight (Fig.1). The highest percentage of rooting for cuttings propagated in the absence of the hormone was achieved with the substrate FCF (67%).
In general, the accession CA-93 presented the lowest average rooting percentage (31%) among the accessions analyzed and the treatments that showed notable averages for the estimated variables were related either to the absence of the hormone or the substrate FCF or FCFCS (Tab.4, Fig.1).
In conclusion, one could say that for the vegetative propagation of L. origanoides, the exogenous application of IBA may not be necessary or its application may be required in low amounts. Also, the substrates FCF and FCFCS produced better results for the variables tested. These results are interesting because the situation is different for other plant species where the exogenous application of auxin is important for growth and plant development, with stimulation of adventitious rooting of cuttings (Campana and Ochoa, 2007; Hartmann et al., 2002).
Figure 2 compares the overall development of the evaluated accessions of L. origanoides. Fig.2B shows the accession CA-93 with a lower and delayed development in general in the FCF0 treatment, which represented the highest rooting effect on the species. In general, the average rooting percentage observed for the two evaluated accessions of L. origanoides (51% for CA-90 and 31% for CA-93) and the values registered for all the other variables were low relative to the other species tested in this study (Tab.4, Fig.1). In a previous study, several non-domesticated wild species of the genus Lippia from Brazil (L. filifolia, L. glandulosa, L.hermannioides, L. rosella, L. rotundifolia and L. sidoides) also showed a lower rooting capacity regardless of season (dry or wet) and the concentration of auxin, relative to the cultivated species Lippia alba (Pimenta et al., 2007). It is interesting to see that the accession CA-90 of L. origanoides responded better to the treatments than did accession CA- 93, indicating that there may be some heterogeneity among individuals in this species that is worth exploring. It can be concluded that more complex propagation methods may be needed for this species in order to obtain more satisfactory results.
Lippia alba (CA-300)
Tab.3 shows that for L. alba, cuttings propagated in the three substrates showed no significant differences in any of the four variables tested, as opposed to hormone concentration where there was an effect. In general, this species registered the highest values for root length, number of roots and root dry weight among all the species analyzed and in the FCF substrate (Tab.4, Fig.1). It can be seen that a higher percentage of rooting was obtained in cuttings propagated in the absence of the hormone (between 67 to 83%) than in the presence of the hormone (20 to 63%) (Tab.3). In the same way, longer roots were observed when the hormone was not applied. In the absence of the hormone, the highest percentage of rooting was observed in the substrate FCFCS (83%) and longer roots in the substrate FCFCSRS (15.47 cm) (Tab.3). In contrast, the application of hormone promoted the number of roots in the cuttings. Further tests showed that the hormone factor (at level 0 or absence) had the highest effect (23%) on all levels provided by the two factors. Although IBA concentration had no statistically significant effect on root dry weight, it can be seen in Tab.3 that in the substrate FCF and at 2,000 mg L-1 IBA, there was a slight increase over the other treatments, indicating that the use of growth promoters such as IBA in this species may increase and accelerate the formation of adventitious roots in asexual plant propagation (Moreno et al., 2009; Li et al., 2009). Pimenta et al. (2007), in studying the number of roots developed in cuttings of L. alba under several IBA concentrations, observed that the number of roots (maximum observed value of 39.4) increased with the increment of hormone (IBA), a response that is similar to the one obtained in the present study.
In summary, accession CA-300 of L. alba has ease of propagation in the different types of tested substrates with slightly better values in the substrate FCF. L. alba showed a higher percentage of rooting in the absence of the hormone and a higher number of roots formed in the presence of hormone. The results of this study are consistent with previous reports on L. alba which showed that this species has high rates and ease of rooting for different types of substrates (rice hulls, vermiculite, soil and Plantmax®) (Biasi and Costa, 2003) and auxin concentrations (Pimenta et al., 2007).
Genus Tagetes (Asteraceae)
Tagetes zypaquirensis (CA-247)
With a value of 66.3% (Tab.4), T. zypaquirensis was the species that had the best average rooting percentage of the evaluated species and in the tested substrates. Although multiple comparison tests generally showed no significant differences between the substrates and hormone concentrations tested, the FCF0 treatment had the highest average rooting (80%) in the absence of the hormone (Tab.3). Additional tests by independent factor (hormone) showed that the absence of the hormone had the single biggest positive effect (9%) for the three levels tested. Longer roots and larger root dry weight were also observed in the absence of hormone, but a larger number of roots was observed when the hormone was applied. Fig.3 shows the CA-247 accession in the treatments FCF0, FCFCS2000 and FCFCSRS 2000 one month after sowing. In general, during the study, this accession was seen as easily propagated, and in this sense is similar to L. alba, facilitating and not restricting the conditions for asexual reproduction. For the asexual propagation of this species, the substrate FCF may be used because this seems to promote rooting, and auxin may be added to promote the growing of more roots.
Tagetes caracasana (CA-10)
Germination tests were performed for T. caracasana both in the laboratory (Fig.4) and in the greenhouse. In the laboratory, the germination percentage averaged 69% for a period of three weeks after which no more germination was observed. The germination speed index (GSI ) was 4.14 for the laboratory conditions, which is relatively high compared with that observed in the greenhouse environment (GSI = 1.45). Similarly, under greenhouse conditions, the germination percentage was slightly lower (61%). These percentages of germination are relatively high when compared with germination in other wild species of the genus, such as Tagetes lucida, which presents a large number of sterile seeds (Acosta de la Luz et al., 2010). The results of the present study are similar to those obtained for Tagetes minuta, a species also propagated by seeds with germination percentages between 50-60% in cultivation conditions and germination completed in a period between 10 to 15 d (Singh et al., 2003).
Conclusions
The rooting of cuttings of L. origanoides (accessions CA-90 and CA-93), L. alba (accession CA-300) and T. zypaquirensis (accession CA-247) showed overall better rooting percentage in the fine coconut fiber substrate and in the absence of hormone, although there was a larger number of roots with the exogenous application of IBA (at 2,000 mg L-1). For its part, the seeds of T. caracasana, either in controlled laboratory conditions or in the greenhouse, had a relatively short germination time (maximum three weeks) and a relatively high germination percentage (about 70%), as such, the seed propagation method is appropriate for this species. The performance of L. origanoides in the asexual propagation methods evaluated was relatively poor and may require more complex and improved methods to obtain more satisfactory results.
Acknowledgements
The present study was financed by the Ministerio de Agricultura y Desarrollo Rural (MADR) of Colombia (contract 062-2007V7163-51) and the Dirección de Investigaciones of the Universidad Nacional de Colombia, Bogota. Thanks are due to Professor Stanislav Magnitskiy for his valuable contributions to the design of the experiments.
Literature cited
Acosta de la Luz, L., I. Hechevarría S., and C. Rodríguez F. 2010. Estudios preliminares para el establecimiento del cultivo de Tagetes lucida Cav (on line). Rev Cubana Plant Med. 15(1), http://www.bvs.sld.cu/revistas/pla/vol_15_1_10/pla05110.htm; consulted, March, 2013 [ Links ]
Antolinez-Delgado, C.A. and N. Rodríguez L. 2008. Plasticidad fenotípica en Lippia alba y Lippia origanoides (Verbenaceae): respuesta a la disponibilidad de nitrógeno. Acta Biol. Colomb. 13, 53-64. [ Links ]
Besnier, F. 1989. Semillas y tecnología. Ediciones Mundi Prensa, Madrid. [ Links ]
Biasi, L.A. and G. Costa. 2003. PropagaÇão vegetativa de Lippia alba. Cienc. Rural 33, 455-459. [ Links ]
Black, B.L. and R.H. Zimmerman. 2002. Mixtures of coal ash and compost as substrates for high bush blueberry. J. Amer. Soc. Hort. Sci. 127, 869-877. [ Links ]
Camargo P., A.A. and N. Rodríguez L. 2008. Respuestas fenotípicas de Lippia alba y Lippia origanoides (Verbenaceae) a la disponibilidad de agua en el suelo. Acta Biol. Colomb. 13, 131-146. [ Links ]
Campana, B.M.R. and M.J. Ochoa. 2007. Propagación vegetativa o agámica de especies frutales. pp. 133-197. In: Sozzi, G.O. (ed.). Árboles frutales. Ecofisiología, cultivo y aprovechamiento. Editorial Facultad de Agronomía, Universidad de Buenos Aires, Buenos Aires. [ Links ]
Casimiro, I., T. Beeckman, N. Graham, R. Bhalerao, H. Zhang, P. Casero, G. Sandberg, and M.J. Bennett. 2003. Dissecting Arabidopsis lateral root development. Trends Plant Sci. 8, 165-171. [ Links ]
Drewes, F.E. and J. Van Staden. 1990. Germination of Tagetes minuta L. II . Role of gibberellins. J. Plant Growth Regul. 9, 285-291. [ Links ]
Ferreira, A.G., B. Cassol, S. Galli, T. Sales Da Silveira, A.L. Stival, and S.A. Andreoli. 2001. GerminaÇão de sementes de Asteraceae nativas no Rio Grande do Sul, Brasil. Acta Bot. Bras. 15, 231-242. [ Links ]
Frangi, P. and S. Nicola. 2005. Study of propagation by cutting of five species native to South Africa. Acta Hort. 683, 313-317. [ Links ]
García-Pérez, E., J.A. Gutiérrez-Uribe, and S. García-Lara. 2012. Luteolin content and antioxidant activity in micropropagated plants of Poliomintha glabrescens (Gray). Plant Cell Tissue Organ Cult. 108, 521-527. [ Links ]
Gupta, S.K., S.P.S. Khanuja, and S. Kumar. 2001. In vitro micropropagation of Lippia alba. Current Sci. 81, 206-210. [ Links ]
Hartmann, H.T., D.E. Kester, F.T. Jr. Davies, and R.L. Geneve. 2002. Plant propagation: principles and practices. 7th ed. Prentice Hall, Saddle River, NJ . [ Links ]
Hernandez-Lozano, D.C., J. Fontecha-Garcia, A.F. Peralta- Bohórquez, C.E. Quijano-Celis, G. Morales, and J.A. Pino. 2010. Composition of the essential oil from leaves of Tagetes zipaquinensis Hump. et Bonpl., grown in Colombia. J. Essen. Oil Res. 22, 371-372. [ Links ]
Hidalgo M., A. 1995. Evaluación de diferentes métodos para la obtención, in vitro, de callos de marigold, Tagetes erecta L. Undergraduate thesis. Faculty of Agricultural Sciences. Universidad Nacional de Colombia, Medellín, Colombia. [ Links ]
Hinesley, L.E., F.A. Blazich, and L.K. Snelling. 1994. Propagation of Atlantic white cedar by stem cuttings. HortScience 29, 217-219. [ Links ]
Juliani, H.R., A.R. Koroch, and V.S. Trippi. 1999. Micropropagation of Lippia junelliana (Mold.) Tronc. Plant Cell Tissue Organ Cul. 59, 175-179. [ Links ]
Kaplan, L. 1958. Historical and ethnobotanical aspects of domestication in Tagetes. Econ. Bot. 14(3), 200-202. [ Links ]
Li, S.W., L.G. Xue, S.J. Xu, H.Y. Feng, and L.Z. An. 2009. IBA-induced changes in antioxidant enzymes during adventitious rooting in mung vean seedlings: The role of H2O2. Environ. Exp. Bot. 66, 442-450. [ Links ]
Mariotti, I., M. Marotti, R. Piccaglia, A. Nastri, S. Grandi, and G. Dinelli. 2010. Thiophene occurrence in different Tagetes species: agricultural biomasses as sources of biocidal substances. J. Sci. Food Agric. 90, 1210-1217. [ Links ]
Maynard, B.K. and N.L. Bassuk. 1990. Rooting softwood cuttings of Acer griseum: promotion by stock plant etiolation, inhibition by cathecol. HortScience 25, 200-202. [ Links ]
Meneses, R., F.A. Torres, E.E. Stashenko, and R.E. Ocazionez. 2009. Aceites esenciales de plantas colombianas inactivan el virus del dengue y el virus de la fiebre amarilla. Salud UIS 41, 236-243. [ Links ]
Misra, P. and S.K. Datta. 2001. Direct differentiation of shoot buds in leaf segments of white marigold (Tagetes erecta L.). In Vitro Cell Dev. Biol. Plant 37, 466-470. [ Links ]
Mohamed, M.A.H., P.J.C. Harris, and J. Henderson. 1999. An efficient in vitro regeneration protocol for Tagetes minuta. Plant Cell Tissue Organ Cul. 55, 211-215. [ Links ]
Moreno, N., J.G. Álvarez-Herrera, H.E. Balaguera-López, and G. Fischer. 2009. Propagación asexual de uchuva (Physalis peruviana L.) en diferentes sustratos y a distintos niveles de auxina. Agron. Colomb. 27, 341-348. [ Links ]
Moreno, F., G.A. Plaza, and S.V. Magnitskiy. 2006. Efecto de la testa sobre la germinación de semillas de caucho (Hevea brasiliensis Muell.). Agron. Colomb. 24, 290-295. [ Links ]
Musarurwa, H.T., L. Koegelenberg, and N.P. Makunga. 2012. Chemical variation in essential oil profiles detected using headspace solid-phase microextraction gas chromatography spectrometry in response to potassium, nitrogen, and water available to micropropagated plants of Salvia stenophylla (Burch. ex Benth.). J. Plant Growth Regul. 31, 207-220. [ Links ]
Nerio, L.S., J. Olivero-Verbel, and E.E. Stashenko. 2009. Repellent activity of essential oils from seven aromatic plants grown in Colombia against Sitophilus zeamais Motschulsky (Coleoptera). J. Stored Prod. Res. 45, 212-214. [ Links ]
Parra, T.E. and N. Rodríguez L. 2007. Plasticidad fenotípica de Lippia alba y Lippia origanoides (Verbenaceae) en respuesta a la disponibilidad de luz. Acta Biol. Colomb. 12S, 91-102. [ Links ]
Pascual, M.E., K. Slowing, E. Carretero, D. Sánchez, and A. Villar. 2001. Lippia: traditional uses, chemistry and pharmacology: a review. J. Ethnopharmacol. 76, 201-214. [ Links ]
Paternina Q., M.J. 2009. Evaluación de la adaptación al medio ambiente de plantas promisorias medicinales, aproximación a los requerimientos ambientales de seis especies de plantas aromáticas medicinales en el departamento del Cauca. Undergraduate thesis. Faculty of Agronomy, Universidad Nacional de Colombia, Bogota. [ Links ]
Peer, W.A. and A.S. Murphy. 2007. Flavonoids and auxin transport: modulators or regulators? Trends Plant Sci. 12, 556-563. [ Links ]
Pereira Peixoto, P.H., F.G. Gonc, A. Salimena, M. De Oliveira Santos, L. Da Silva Garcia, P.M. De Oliveira Pierre, L.F. Viccini, and W. Campos-Otoni. 2006. In Vitro Propagation of endangered Lippia filifolia Mart. and Schauer Ex Schauer. In Vitro Cell. Dev. Biol. Plant 42, 558-561. [ Links ]
Pimenta, M.R., L.S. Fernandez, U.J. Pereira, L.S. Garcia, S.R. Leal, S.G. Leitão, F.R.G. Salimena, L.F. Viccini, and P.H.P. Peixoto. 2007. Floração, germinação e estaquia em espécies de Lippia L. (Verbenaceae). Rev. Bras. Bot. 30, 211-220. [ Links ]
Quintero, M.F., C.A. González, and J.M. Guzmán, 2011. Sustratos para cultivos hortícolas y flores de corte. pp. 79-108. In: Flórez R., V.J. (ed.). Sustratos, manejo del clima, automatización y control en sistemas de cultivo sin suelo. Universidad Nacional de Colombia, Bogota. [ Links ]
Rojas P., D. 1994. Evaluación de la respuesta del marigold Tagetes erecta L. a diferentes sistemas y dosis de fertilización. Undergraduate thesis. Faculty of Agricultural Sciences, Universidad Nacional de Colombia, Medellín, Colombia. [ Links ]
Sauerwein, M., H.E. Flores, T. Yamazaki, and K. Shimomura. 1991. Lippia dulcis shoot cultures as a source of the sweet sesquiterpene hernandulcin. Plant Cell Rep. 9, 663-666. [ Links ]
Seaforth, C. and T. Tikasingh. 2008. A study for the development of a handbook of selected Caribbean herbs for industry. Technical Centre for Agriculture and Rural Cooperation (CTA); Inter- American Institute for Cooperation on Agriculture (II CA). Wageningen, The Netherlands; St. Augustine, Trinidad. [ Links ]
Singh, V., B. Singh, and V.K. Kaul. 2003. Domestication of wild marigold (Tagetes minuta L.) as a potential economic crop in western Himalaya and north Indian plains. Econ. Bot. 57, 535-544. [ Links ]
Stashenko, E.E., J.R. Martínez, C.A. Ruiz, G. Arias, C. Durán, W. Salgar, and M. Cala. 2010. Lippia origanoides chemotype differentiation based on essential oil GC-MS and principal component analysis. J. Sep. Sci. 33, 93-103. [ Links ]
Struve, D.K. and B.C. Moser. 1984. Auxin effects on root regeneration of scarlet oak seedlings. J. Amer. Soc. Hort. Sci. 109, 91-95. [ Links ]
Vanegas, P.E., A. Cruz-Hernández, M.E. Valverde, and O. Paredes- López. 2002. Plant regeneration via organogenesis in marigold. Plant Cell Tissue Organ. Cul. 69, 279-283. [ Links ]
Wasson, A.P., A.I. Pellerone, and U. Mathesius. 2006. Silencing the flavonoid pathway in Medicago truncatula inhibits root nodule formation and prevents auxin transport regulation by rhizobia. Plant Cell 18, 1617-1629. [ Links ]





![Stomatal behavior in fruits and leaves of the purple passion fruit (Passiflora edulis Sims) and fruits and cladodes of the yellow pitaya [Hylocereus megalanthus (K. Schum. ex Vaupel) Ralf Bauer]](/img/en/next.gif)