Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Agronomía Colombiana
versão impressa ISSN 0120-9965
Agron. colomb. vol.31 no.2 Bogotá maio/ago. 2013
PROPAGATION & TISSUE CULTURE
Sugar apple (Annona squamosa L., Annonaceae) seed germination: morphological and anatomical changes
Germinación de semillas de anón (Annona squamosa L., Annonaceae): cambios morfológicos y anatómicos
Fabio Ernesto Martínez-Maldonado1 Diego Miranda-Lasprilla2 and Stanislav Magnitskiy2
1Research Center Tibaitatá, Corporación Colombiana de Investigación Agropecuaria (Corpoica). Mosquera (Colombia). femartinezma@gmail.com2Department of Agronomy, Faculty of Agronomy, Universidad Nacional de Colombia. Bogota (Colombia).
Received for publication: 6 February, 2013. Accepted for publication: 5 June, 2013.
ABSTRACT
The anon or sugar apple is a species of the Annona genus, widely distributed in the world and in Colombia and a fruit with great potential in domestic and international markets. However, the technical information related to the aspects of propagation and production is limited. In the present study, the morphological and anatomical changes during seed germination of the sugar apple were determined using histological techniques and photographic records. The results show that seed germination is a process that takes place in two stages: testa rupture and endosperm rupture-radicle protrusion. In the post-germination stages, the induction and formation of lateral roots that were endogenously produced from the primary root from the pericycle were seen. The endosperm underwent morphological changes that increased its volume during imbibition and degraded in the final stages of germination, which could be indicative of endosperm weakening and reduction of mechanical strength imposed by embryo growth, which was required to complete germination in A. squamosa.
Key words: testa, endosperm, radicle, roots.
RESUMEN
El anón, es la especie del género Annona más ampliamente distribuida en el mundo y en Colombia es un fruto con gran potencial en mercados nacionales e internacionales. Sin embargo la información técnica relacionada con aspectos de propagación y producción es limitada. En el presente estudio mediante técnicas histológicas y registros fotográficos fueron determinados los cambios morfológicos y anatómicos durante la germinación y de semillas de anón. Los resultados muestran que la germinación de las semillas es un proceso que se desarrolla en dos etapas consecutivas: la ruptura de la testa y la ruptura del endospermo - protrusión radicular. En las etapas de post-germinación se presenta la formación e inducción de raíces laterales producidas de forma endógena desde la raíz primaria a partir del periciclo. El endospermo sufre evidentes cambios morfológicos, incrementando su volumen en la etapa de imbibición y degradándose en etapas finales del proceso de germinación, lo que podría ser indicativo del debilitamiento del endospermo, y disminución de la resistencia mecánica impuesta por este al crecimiento de embrión, la cual es necesaria para que la germinación se complete en A. squamosa.
Palabras clave: testa, endospermo, radícula, raíces.
Introduction
The Anon or sugar apple Annona squamosa L. is a species of the Annona genus which is widely distributed in the world. In Colombia, it is found in the dry areas of the valleys in the provinces of Valle, Caldas, Tolima, Cundinamarca, and Santander (Guerrero and Fischer, 2007). The sugar apple grows from sea level to 1,000 m and may not support cold periods, developing and growing well in relatively stable temperature conditions (George and Nissen, 1987). The minimum temperature is in a range of 10 to 20°C and the maximum temperature has a range of 22 to 28°C (Guerrero and Fischer, 2007).
The seeds of Annonaceae are albuminous ellipsoids and their length varies between 5 and 30 mm. They have a ruminate endosperm (Corner, 1976; Garwood, 1995; Judd et al., 1999). The embryo is small, straight, with moderately developed embryonic axis, rudimentary plumule and flat and thin cotyledons; and develops after the seed is formed (Corner, 1976; Garwood, 1995, Judd et al., 1999). Setten and Koek-Noorman (1992) indicated that Annonaceae seeds undergoing dispersal have a small embryo that is considered underdeveloped and immature; immaturity requires time to complete embryo growth after seed dispersion. Meanwhile, Hayat (1963) reported that the seeds of A. squamosa have a small embryo with two foliaceous, thin cotyledons that take one to three months to germinate.
Germination could be defined as the emergence and development from the embryo of all the essential structures that are indicative of the ability of the seed to produce a normal seedling under favorable conditions (AOSA , 2000). According to Bewley (1997) and Finch-Savage and Leubner-Metzger (2006), the germination of seeds is a complex physiological process caused by water imbibition after possible mechanisms of latency have been overcome. Under favorable conditions, the rapid growth of the embryo breaks the seed coat layers and presents the protrusion of the radicle. This emergence of the radicle is regarded as completion of germination.
This transition point (radicle protrusion) is also characterized by a loss of desiccation tolerance and a molecular checkpoint (in Arabidopsis this is regulated by ABI5), while a molecular switch generates the transition development program from germination to the seedling development program (Bewley and Black, 1994).
At the completion of germination, radicle protrusion depends on embryo growth, a process led by water uptake. This water uptake is a triphasic process with a rapid initial uptake (imbibition phase I) followed by a stabilization phase (phase II) and a late phase III, which has an increase in water uptake and that occurs only when germination is completed, once the embryo has elongated the axis and traversed the coat structures (Schopfer and Plachy, 1984; Bewley, 1997; Manz et al., 2005). The necessary cell elongation has been generally accepted as sufficient to generate the protrusion of the radicle, while cell division in many species is considered nonessential.
In these phases, cellular metabolism is increased and the embryo resumes active growth, forcing the breaking of the seed coat to allow radicle emergence (Bewley, 1997). In early stages of germination, the imbibition process occurs in which the seed coat weakens, hydrating the protoplasm, which swells the seed to the point of testa rupture (Crowe et al., 1998).
Besides this physical process, the enzyme and protein synthesis system is activated by the seed water absorption, which allows the formation of substances necessary for development. During the second phase, water absorption and respiration are ongoing processes (Bewley and Black, 1994); simultaneously, starch, lipids, and proteins in the endosperm are hydrolyzed to sugars, fatty acids and amino acids, simple compounds that are soluble and mobile. Subsequently, these substances are mobilized to the growing points of the embryonic axis and are used in growth processes, which allow the growth of the radicle of the embryo (Sierra, 2005).
For many species, including Arabidopsis sp., Nicotiana sp., Lepidium sativum, and Petunia hybrida, germination is a process that occurs in two stages: the first stage corresponding to testa rupture and the second one corresponding to endosperm rupture, two sequential events separated in time during germination (Petruzzelli et al., 2003, Leubner- Metzger, 2003; Liu et al., 2005). In endospermic seeds, both the testa and the endosperm may contribute to physical dormancy (Hilhorst, 1995; Bewley, 1997; Leubner-Metzger, 2003). The endosperm may be considered as the main obstacle to the germination of seeds of Asteraceae (lettuce), Solanaceae (tomato and tobacco) and Rubiaceae (coffee). In these cases, a weakened micropylar endosperm is required for radicle protrusion and is associated with cell wall hydrolysis by hydrolytic enzymes (Hilhorst, 1995; Bewley, 1997; Koornneef et al., 2002; Leubner-Metzger, 2003). This study aimed to identify the stages of germination of A. squamosa seeds and describe some changes in seed anatomy and morphology during germination. The knowledge of morphological changes in A. squamosa seeds during germination could further contribute to the understanding of physiological and biochemical events during seed germination and plantlet establishment in this species.
Materials and methods
Plant material
The selected material was part of the accessions collected for project development in the Colombian Bank of Anon Germplasm, framed within the program: Planting Material and Breeding of the sugar apple. The accessions selected were collected in 2011 in the municipality of Apulo, Cundinamarca; plant individuals were distributed in agroecosystems and not in the field or spontaneously in gardens. The seeds of A. squamosa were obtained from fruits of recollected accessions C2AS 224 (C: Cundinamarca; 2: municipality code, AS : species A. squamosa; 224: number of row), C2AS 226, and C2AS 225. From fruits, ripe and soft to the touch, that were washed with room temperature water to remove any residue present on the surface, the exocarp was separated from the mesocarp (pulp). Subsequently, the seeds were removed manually from the pulp and aryl, after which these were washed with water and placed on absorbent paper towels at room temperature in order to dry to moisture contents of 10%. The seeds were disinfected with 1% sodium hypochlorite for 9 min and afterwards washed with 96% ethanol and washed three times with distilled water.
Morphological analysis and preparation of the material For the morphological analysis, the following treatments were used: 1) untreated, ripe anon seeds before germination with 10% moisture content, 2) seeds imbibed in distilled water for 72 h, 3) germinating seeds with seed coat rupture, 4) seeds with ruptured endosperm and radicle protrusion, and 5) seeds with a radicle length > 3 mm (lateral root initiation). Seed germination was performed on peat substrate trays (Klassmann® without nutrients, Geeste, Germany,) with seeds located at a depth equal to twice the length and that were subsequently placed in a Phytotron chamber, Conviron® CMP3244, for 30 d at 35°C, in the absence of light as seeds of A. squamosa are indifferent to light conditions for germination (Ferreira et al., 1997). The observations of the morphological changes in the seeds were performed using a Leica® stereoscope zoom 2000 (Solms, Germany), using 30 ungerminated seeds, imbibed seeds, seeds with ruptured seed coat, seeds with ruptured testa and endosperm, and seeds with radicle protrusion. A photographic record of the seeds was made for each state.
Anatomical analysis
Untreated, whole A. squamosa seeds with 10% moisture and fragments of imbibed radicles with lateral roots were immersed in Bouin's fixative (Bouin, 1897) for 48 h at room temperature to stop the deterioration of the tissues. The cassettes with the fragments were removed from the fixing solution and were passed through a concentration gradient of ethanol of 65, 70, 75, 80, 85, 90, 96 and 96%, each for a period of 24 h. Subsequently, the tissues were immersed for 24 h in absolute alcohol and after that, they were imbibed in tert-butanol for 24 h. The sections were imbibed for 24 h at 60°C in 4 graded concentrations of molten paraffin at 56°C and tert-butanol, increasing the concentration of paraffin and decreasing the concentration of tert-butanol, thus providing: a) 25% paraffin - 75% tert-butanol, b) 50% paraffin - 50% tert-butanol, c) 75% paraffin - 25% tert-butanol, and d) 100% paraffin. The blocks were prepared and, prior to the final cutting, were immersed in a solution of 50% hydrochloric acid for 2 h. Afterwards, they were washed with water and placed on ice in order to increase the strength of the paraffin. The cuts were made in a microtome 7 µm. with a brush; the sections were placed in a water bath at 50°C. The sections were captured using a carrier sheet that was previously treated with Mayer albumin. Subsequently, the sections were dewaxed by placing them in an oven at 60°C for 24 h. And then to completely remove the paraffin from the sheets, they were passed through xylol for 10 min two times and then two times for 10 min in 96% ethanol. The staining was carried out over 24 h with safranin and fast green. Once the dyeing process was performed, the sheets were cleaned and historesine was applied over the cuts with a foil sealing coverglass. The documentation and observations were made with an Olympus® CX31 microscope (Tokyo, Japan) and the image editing software Image-Pro Plus® (Rockville, MD).
Results and discussion
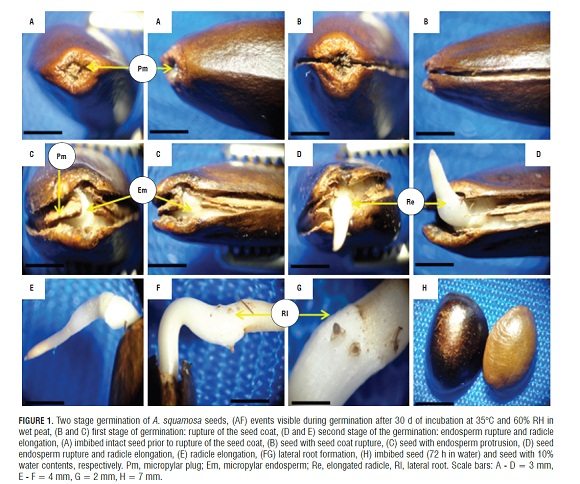
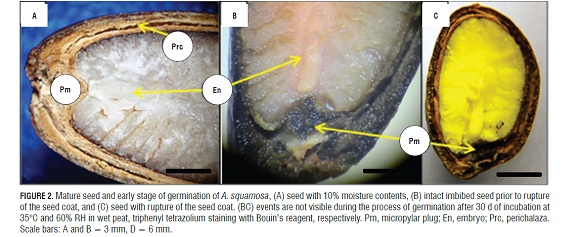
The germination of mature seeds of A. squamosa is a process that occurred in two separate and consecutive stages: the rupture of the testa and, later, the rupture of the endosperm (Fig.1A, 1B, and 1D). The mature, dry seeds had a slightly developed embryo but distinct tissues, where the micropylar plug, the embryo and the endosperm were easily distinguishable. Likewise, a rupture region of the seed coat that constitutes the perichalaza region may be observed (Fig.2A and 3A).
During imbibition, the seeds expanded and there were changes in size (Fig.1H). The germination process started with rapid water absorption (phase I, imbibition), which occurred during the first 17.6 h of the 72 h imbibition, where, in comparison with the dry seeds (Fig.1A), the seeds had an expanded embryo (Fig.2b), then the first stage of germination of the seed coat rupture presented (Fig. 1B ), a pregerminative event that happened without any treatment between 10 and 15 d of incubation. This rupture in the seed coat was due to the increase in the volume of the endosperm and embryo size (Fig.2C). The testa rupture started at the micropylar plug, consisting of a thin, endotestal tissue and a well-defined region of fracture (Fig.2A AND B). Then, between 15 and 20 d of incubation, the second stage of germination with protrusion and endosperm rupture presented, demonstrating how residues were expelled through the micropylar plug (Fig.1C and 4C), and, consequently, the protrusion of the radicle occurred (Fig.1D and 4B) which strictly might be called germination. The phase of water absorption continued during the transition towards plumule and radicle growth (even within the seed) that presented in the lateral root formation (phase III of water uptake) (Fig.1F, 1GFigs. 1F, 1G, and 3C).
Reports of germination in two steps, as described for A. squamosa, were found for Arabidopsis sp. (Liu et al., 2005), Lepidium sp. (Muller et al., 2006), and tobacco (Liu et al., 2005; Manz et al., 2005; Muller et al., 2006). This condition of germination has also been described in Trollius sp. (Ranunculaceae; Hepher and Roberts, 1985), Chenopodium sp.(Amaranthaceae; Karssen, 1968; Karssen, 1976), Nicotiana sp. and Petunia sp. (Cestroideae subfamily of Solanaceae, Leubner-Metzger et al., 1995; Krock et al., 2002; Petruzzelli et al., 2003).
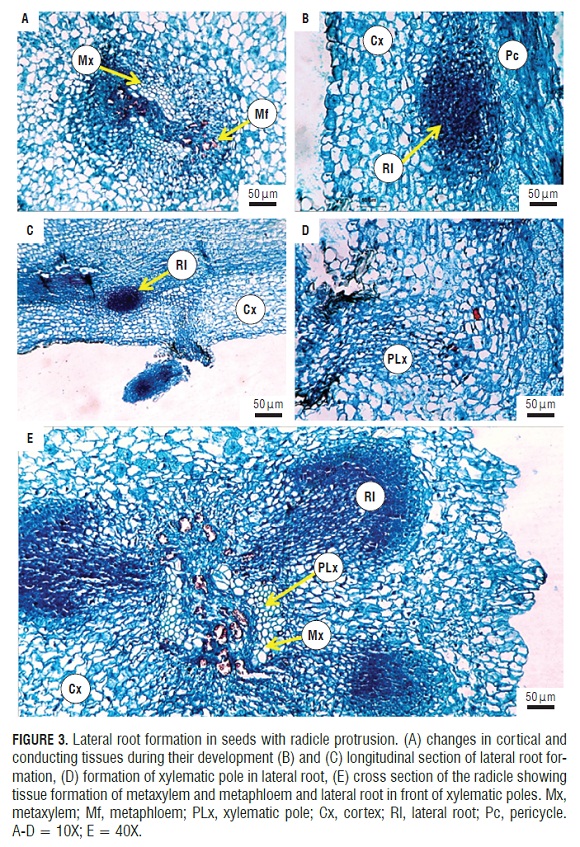
In post-germination (Fig.1F), the formation and induction of lateral roots were seen. These endogenously appeared in the radicle from the pericycle (Fig.3B) and were initiated by anticlinal and periclinal divisions in a group of cells that formed the lateral root primordia, which grows and penetrates the cortex. FigS.3A and 3B show the start of the divisions in the pericycle. One can see the formation of lateral roots in front of xylem poles that are evident in the cut (Fig.3E). It can be seen how the lateral root primordia was developing and growing through the cortex and epidermis of the primary root (Fig.3C,3D,and 3E), which was achieved by enzymatic lisis or simple mechanical action (Alonso, 2011). Initially, there was a connection between the vascular cylinder of the primary root and lateral root. The literature data support the conclusion that the connection is made through transition cells derived from the pericycle or parenchyma, connecting both vascular cylinders. These transition cells are called interposed cells that are differentiated from the sieve elements or xylem vessels (Alonso, 2011).
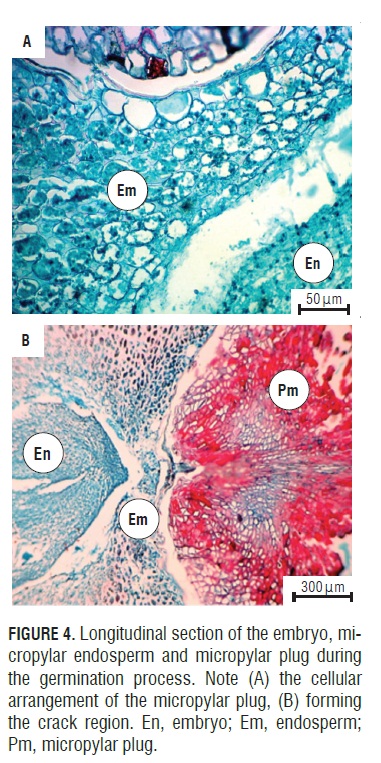
The anatomical study showed how the seeds of A. squamosa presented the endosperm with cell wall thickening (Fig.4A), which is due to galactomannans that are deposited on the cell walls (Buckeridge et al., 2000). However, in the micropylar region, thin walls could be seen. This change occurred because the endosperm in this area may be less resistant to the radicle protrusion generated by the growth of the embryo (Da Silva et al., 2007).
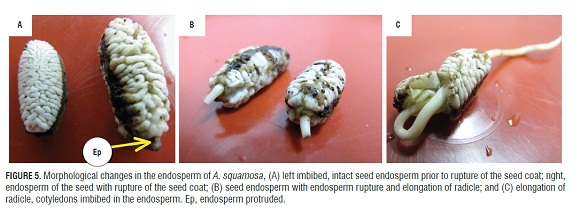
Figure 5 shows seeds without testa, where the endosperm can be appreciated after imbibition (Fig.5A RIGHT), in the seed coat rupture step (in which the endosperm can be seen protruding) (Fig.5A LEFT), in the endosperm and the disruption of the radicle protrusion step (Fig.5B), and in postgermination growth (Fig.5C). The change in size and shape of the endosperm and its protrusion through the rupture of the seed coat could be indicative of endosperm weakening and, therefore, a reduction in mechanical strength, which is necessary to complete germination in A. squamosa. This is supported by reports by Martínez (2012), who found that these seeds have a morphophysiological dormancy, where, together with low growth potential of the embryo, there are restrictions for tissue growth due to the endosperm tissue and testa (Bewley, 1997; Bradford et al., 2000; Hegashi et al., 2002; Taiz and Zeiger, 2006).
In Trollius sp. (Hepher and Roberts, 1985), Chenopodium sp. (Karssen, 1976), several solanaceas, such as the tomato and tobacco, and Asteraceae, such as lettuce (Hilhorst, 1995; Bewley 1997; Leubner-Metzger, 2003), the endosperm acts as a barrier to the protrusion of the radicle and likewise for the completion of angiosperm seed germination. The weakening of the endosperm could be related to the activity of the endo-Β-mannanase enzyme, which is associated with hydrolysis of the galactomannans contained in cell walls of many species including Datura ferox (Sánchez and de Miguel, 1997), Solanum lycopersicum (Groot et al., 1988) and Coffea arabica (da Silva et al., 2004). The authors agree that, as a result of the weakening of the endosperm, carbohydrates are released that constitute an energy source for the growing embryo and mechanical constraints are reduced to radicle growth. In this regard, Bewley (1997) noted that endo-Β-mannanase is involved in endosperm weakening, although Β-1,3-glucanase, in response to light, gibberellins, ethylene and ABA, has been implicated as a regulator of germination (Leubner-Metzger and Meins, 1999).
In seeds of Annona crassiflora, the beginning of embryo growth coincides with the appearance of endo- Β-mannanase around the cavity in the region of the embryonic micropylar endosperm and subsequently diffuses into the lateral endosperm. Endo- Β -mannanase serves to degrade the endosperm to provide energy-rich compounds in the embryo and also to create a space in the cavity in order to accommodate the embryo during embryo growth before radicle protrusion (da Silva et al., 2004). In seeds of A. squamosa, endosperm weakening could be associated with the action of hydrolytic enzymes. Evidence of this is seen in the positive effect of an exogenous application of giberrelic acid on germination variables found by Martínez (2012). With the application of gibberellins, the weakening of the endospermic tissue is promoted (Bewley, 1997; Bradford et al., 2000; Hegashi et al., 2002; Taiz and Zeiger, 2006) due to the synthesis of various hydrolytic enzymes involved in the solubilization of reserves, which include a- and Β-amylase (Taiz and Zeiger, 2006).
Conclusions
The germination of mature A. squamosa seeds is a process that occurs in two separate and consecutive stages: the rupture of the testa and the rupture of the endosperm that occurs later. During post-germination, the induction and formation of lateral roots are presented. These occur endogenously in the primary root from the pericycle.
The weakening of the endosperm starts after the change in size and shape of this structure, in the rupture of the coat (the first stage of germination) and at the beginning of the second stage of germination, wherein the endosperm is observed to be protruded due to radicle growth, despite the micropylar plug residues that remain.
Acknowledgements
The authors express their gratitude to the Graduate School of the Faculty of Agronomy, Universidad Nacional de Colombia, Bogota, for help in developing this study.
Literature cited
Alonso, J.R. 2011. Manual de histología vegetal. 2nd ed. MundiPrensa, Madrid. [ Links ]
AOSA , Association of Official Seed Analysts. 2000. Rules for testing seeds. Bozeman, MT . [ Links ]
Bewley, J.D. and M. Black. 1994. Seeds: physiology of development and germination. 2nd ed. Plenum Press, New York, NY . [ Links ]
Bewley, J.D. 1997. Seed germination and dormancy. Plant Cell. 9, 1055-1066. [ Links ]
Bouin, P. 1897. Phenomenes cytologique anoramoux dans. 1 Histogenes, Nancy, France. [ Links ]
Bradford, K.J., F. Chen, M. Cooley, P. Dahal, B. Downie, K. Fukunaga, O. Gee, S. Gurusinghe, R. Mella, H. Nonogaki, and K. Yim. 2000. Gene expression prior to radicule emergence in imbibed tomato seeds. pp. 231-251. In: Black, M., K. J. Bradford, and J. Vazquezramos (eds.). Seed biology: advances and applications. CAB International, Wallingford, UK. [ Links ]
Buckeridge, M., H. Dos Santos, and M. Tíne. 2000. Cell wall storage polysaccharides in seeds. Structure, metabolism, function and ecological aspects. Plant Physiol. Biochem. 38, 141-156. [ Links ]
Corner, E.J.H. 1976. The seeds of dicotyledons, 1 and 2. 1st ed. University Press, Cambridge. [ Links ]
Crowe, J.H., J.F. Carpenter, and L.M. Crowe. 1998. The role of vitrification in anhydrobiosis. Annu. Rev. Physiol. 60, 73-103. [ Links ]
da Silva, E., D. De Melo, A.C. Davide, N. De Bode, G.B. Abreu, J.M.R. Faria, and H.W.M. Hilhorst. 2007. Germination ecophysiology of Annona crassiflora seeds. Ann. Bot. 99, 823-830. [ Links ]
da Silva, E., P. Toorop, A. Van Aelst, and H. Hilhorst. 2004. Abscisic acid controls embryo growth potential and endosperm. The plant cellcap weakening during coffee (Coffea arabica cv. Rubi) seed germination. Planta 220, 251-260. [ Links ]
Ferreira, G., E. Cereda, C. Silva, R. Cunha, and A. Cataneo. 1997. Imbibition studies of sugar apple (Annona squamosa L.) and atemoya (Annona Hibrid) seed. pp. 210-225. In: Memorias del II Congresso International de Anonaceas. Universidad Autonoma Chapingo (UA C), Chapingo, Mexico. [ Links ]
Finch-Savage, W.E. and G. Leubner-Metzger. 2006. Seed dormancy and the control of germination. New Phytol. 171, 501-523. [ Links ]
Garwood, N.C. 1995. Studies in Annonaceae. XX. Morphology and ecology of seedlings, fruits and seeds of selected Panamanian species. Botan. Jahrbüc. System. 117, 1-152. [ Links ]
George, A.P. and R. Niessen. 1987. Propagation of Annona species: a review. Scient. Hort. 33, 75-85. [ Links ]
Groot, S., B. Kieliszewska-Rokicka, E. Vermeer, and C. Karssen. 1988. Gibberellin-induced hydrolysis of endosperm cell walls in gibberellin-deficient tomato seeds prior to radicle protrusion. Planta 174, 500-504. [ Links ]
Guerrero, E. and G. Fischer. 2007. Manejo integrado en el cultivo de anón (Annona squamosa L.). Rev. Colomb. Cienc. Hortic. 1, 154-169. [ Links ]
Hayat, M.A. 1963. Morphology of seed germination and seedling in Annona squamosa. Botan. Gazette 124, 360-362. [ Links ]
Hegashi, E., R. Silveira, C. Golveia, and L. Basso. 2002. Ação fisiológica de hormônios vegetais nas condições hídricas, metabolismo e nutrição mineral. pp. 139-158. In: Castro, P.R.C., J.O.A. Sena, and R.A. Kruge (eds.). Introdução à fisiología do desenvolvimiento vegetal. Editora da Universidade Estadual de Maringá (Eduem), Maringa, Brazil. [ Links ]
Hepher, A. and J. Roberts. 1985. The control of seed germination in Trollius ledebouri: the breaking of dormancy. Planta 166, 314-320. [ Links ]
Hilhorst, H.W.M. 1995. A critical update on seed dormancy. Primary dormancy. Seed Sci. Res. 5, 61-73. [ Links ]
Judd, W.S., C. Campbell, E. Kellogg, and P. Stevens. 1999. Plant Systematics: a phylogenetic approach. Sinauer Associates, Sunderland, MA . [ Links ]
Karssen, C.M. 1968. The light promoted germination of the seeds of Chenopodium album L.; II. Effects of (RS) - abscisic acid. Acta Bot. Neerl. 17, 293-308. [ Links ]
Karssen, C.M. 1976. Uptake and effect of abscisic acid during induction and progress of radicle growth in seeds of Chenopodium album. Physiol. Plant. 36, 259-263. [ Links ]
Koornneef, M., L. Bentsink, L., and H. Hilhorst. 2002. Seed dormancy and germination. Curr. Opin. Plant. Biol. 5, 33-36. [ Links ]
Krock, B., S. Schmidt, C. Hertweck, and I. Baldwin. 2002. Vegetation derived abscisic acid and four terpenes enforce dormancy in seeds of the post-fire annual, Nicotiana attenuata. Seed Sci. Res. 12, 239-252. [ Links ]
Leubner-Metzger, G. 2003. Functions and regulation of β-1,3- glucanase during seed germination, dormancy release and after-ripening. Seed Sci. Res. 13, 17-34. [ Links ]
Leubner-Metzger, G. and F. Meins. 1999. Functions and regulation of plant -1,3-glucanases (PR-2). pp. 49-76. In: Datta, S.K. and S. Muthukrishnan (eds.). Pathogenesis-related proteins in plants. CRC Press, Boca Raton, FL. [ Links ]
Leubner-Metzger, G., C. Frundt, R. Vogeli-Lange, and F. Meins. 1995. Class I β-1,3-glucanase in the endosperm of tobacco during germination. Plant Physiol. 109, 751-759. [ Links ]
Liu, P., N. Koizuka, T. Homrichhausen, J. Hewitt, R. Martin, and H. Nonogaki. 2005. Large-scale screening of Arabidopsis enhancer-trap lines for seed germination-associated genes. Plant J. 41, 936-944. [ Links ]
Manz, B., K. Muller, B. Kucera, F. Volke, and G. Leubner-Metzger. 2005. Water uptake and distribution in germinating tobacco seeds investigated in vivo by nuclear magnetic resonance imaging. Plant Physiol. 138, 1538-1551. [ Links ]
Martínez, F. 2012. Caracterización morfoanatómica de semillas de anón (Annona squamosa L.) y evaluación de algunos parámetros fisiológicos del proceso de germinación y latencia. M.Sc. thesis. Faculty of Agronomy, Universidad Nacional de Colombia, Bogota. [ Links ]
Muller, K., S. Tintelnot, and G. Leubner-Metzger. 2006. Endospermlimited Brassicaceae seed germination: abscisic acid inhibits embryo-induced endosperm weakening of Lepidium sativum (cress) and endosperm rupture of cress and Arabidopsis thaliana. Plant Cell Physiol. 47, 864-877. [ Links ]
Petruzzelli, L., K. Muller, K. Hermann, K., and G. Leubner-Metzger. 2003. Distinct expression patterns of β-1,3-glucanases and chitinases during the germination of solanaceous seeds. Seed Sci. Res. 13, 139-153. [ Links ]
Sánchez, R.A. and L. de Miguel. 1997. Phytochrome promotion of mannan-degrading enzymes in the micropylar endosperm of Datura ferox seeds requires the presence of the embryo and gibberellin synthesis. Seed Sci. Res. 7, 27-33. [ Links ]
Schopfer, P. and C. Plachy. 1984. Control of seed germination by abscisic acid. II. Effect on embryo water uptake in Brassica napus L. Plant Physiol. 76, 155-160. [ Links ]
Setten, K. and J. Koek-Noorman. 1992. Fruits and seeds of Annonaceae. Morphology and its significance for classification and identification. Bibliot. Botan. 142, 1-101. [ Links ]
Sierra, J. 2005. Fundamentos para el establecimiento de pasturas y cultivos forrajeros. Editorial Universidad de Antioquia, Medellin, Colombia. [ Links ]
Taiz, L. and E. Zeiger. 2006. Plant physiology. 3rd ed. Artmed, Porto Alegre, Brazil. [ Links ]